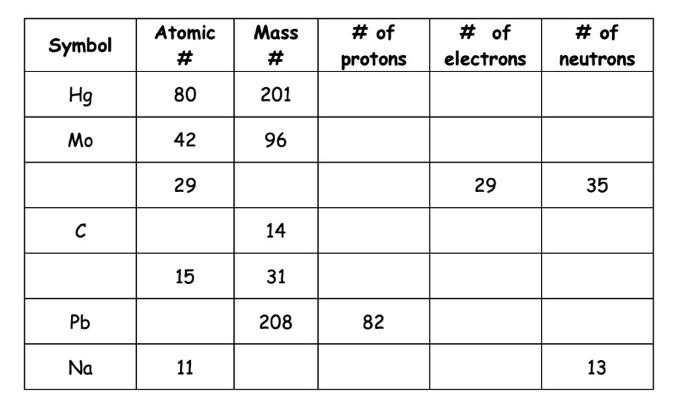
How many sig figs?
0.0009873
4
The zeros at the front do not count!
DRAW OUT CONVERSION
1000g= 1kg
3.9kg
What is the first group on the Periodic Table called?
Group A1: ________
Alkali Metals
What gives an atom its identity?
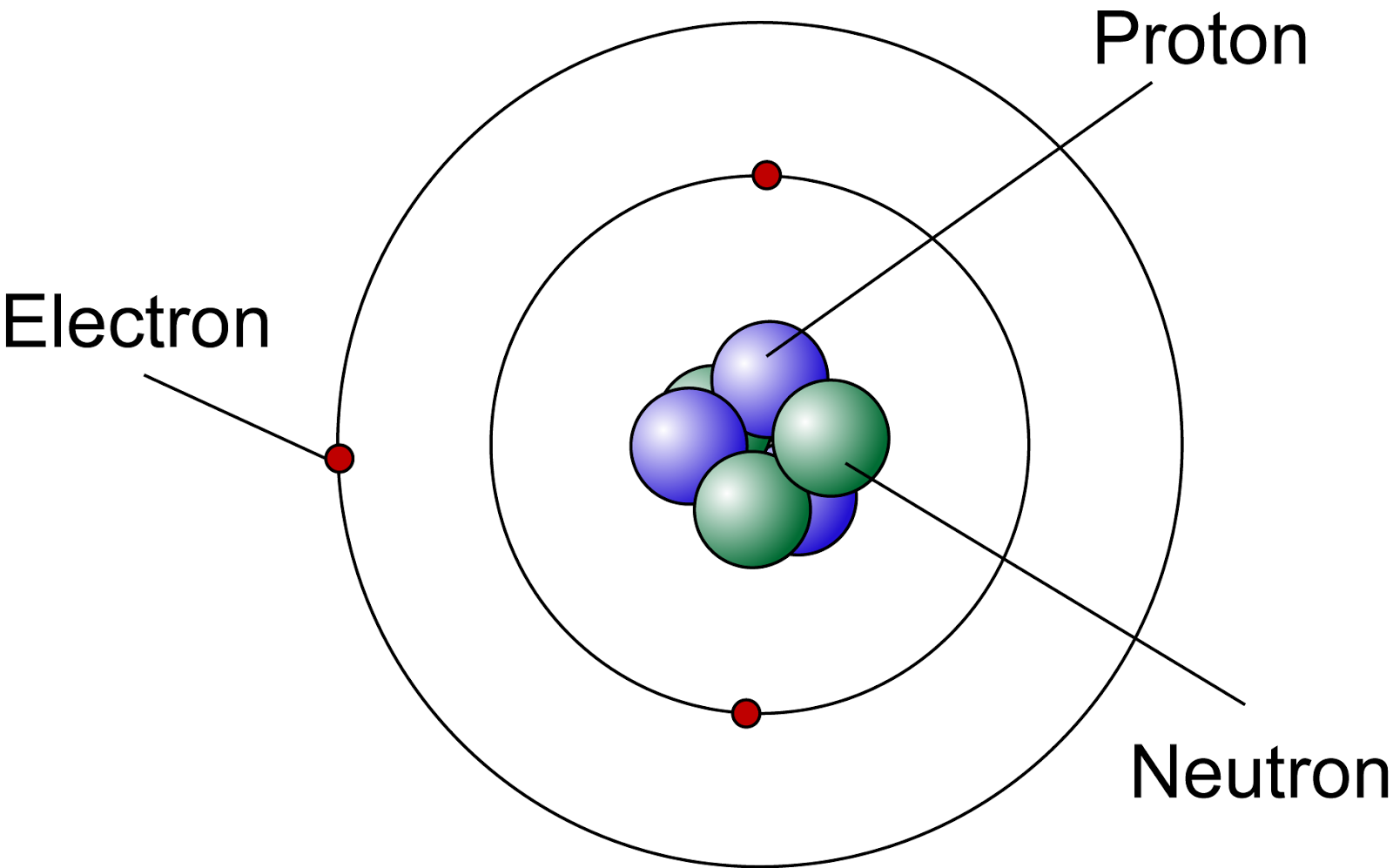
Atomic # aka The number of PROTONS
Give me an example of physical vs. chemical change

a. 0.000067543
b. 3.907
c. 5.48
d. 67.08
c. 5.48
There are 3 sig figs!
Tell me why the rest aren't...
Convert 3.00 x 10^6 miles into meters
1 miles= 1609.3 meters
4.83 x 10^9 meters
What is the second group on the Periodic Table called?
Group 2A: ________
What is the seventh group on the Periodic Table called?
Group 7A: ________
Pt. 1: Alkaline earth metals
Pt. 2: Halogens
How many protrons are in an neutral Ca atom?
20 protrons
What is the lowest state of energy something can be in? AND WHY??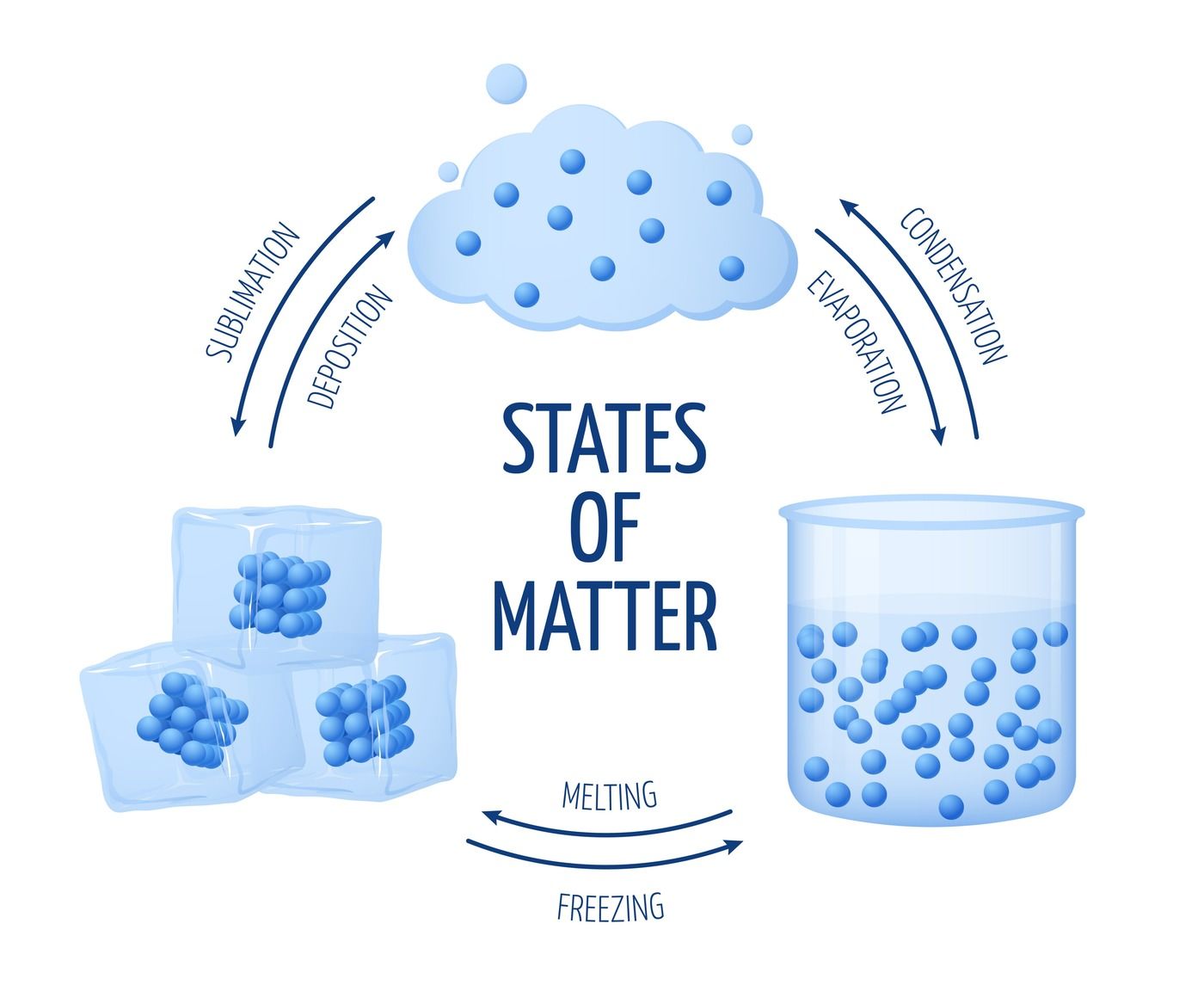
Solids!
12.98 + 7.0= _________
and write the correct sig figs!
20.0
12.98 = 2 decimal places
7.0 = 1 decimal places
Add together!
Convert 120 degrees C into degrees F
°Fahrenheit = (9/5) °Celsius + 32
Pt. 1: What is the eighth group on the Periodic Table called?
Group 8A: ________
Pt. 2: What element is in ROW 3 PERIOD 6
Pt. 1: Nobel Gases
Pt. 2: Sulfur (S)
How many protrons, neurtons, and electrons are in a neutral Cl atom? How does that relate to the atomic mass?
17, 17, and 17
Atomic Mass of Cl = 35.45 (p + n)
Define an Isotope...
same # of protons, differing in neutrons
23.982 x 0.035 = __________
and write the correct sig figs!
0.84
Convert 8.23 x 10^8 m/s into mph
(HINT- how many steps?)
3600 seconds = 1 hour
1 mile = 1609.3 meters
1.84 x 10^9
Name the 3 main groups of elements and where they are found on the Periodic Table
1. Metals (Middle D-block)
2. Metalloids (Diagnol from Boron for the most part)
3. Nonmetals (Hydrogen + top right corner)
What does the numbers on the top and bottom represent?
4 = atomic mass
2 = atomic # (protons)
What state is your AWESOME SI originally from???

Illinois!
Tell me how many sig figs in EACH number and EXPLAIN the rules for each
a. 12.0
b. 1205
c. 1200
a. 3 (Look at decimal)
b. 4 (Zero is inbetween)
c. 2 (No decimal)
The density of Maddie's secret mixute is 123.98g/mL. What is the volume of 34kg of her mixture?
D = M/V
274.24mL
Explain each part of the blocks on the Periodic table and what they mean
Atomic number (protons), Atomic Mass (nucleus), Atomic Symbol (C for Carbon), and Name
Complete the empty row for lead
Pb
#82
p = 82
e = 82
n = 126
Who created the Periodic Table of Elements?
Dmitri Mendeleev