Scientific Inquiry
Scientific Inquiry
Diversity of Life
Large Biological Molecules
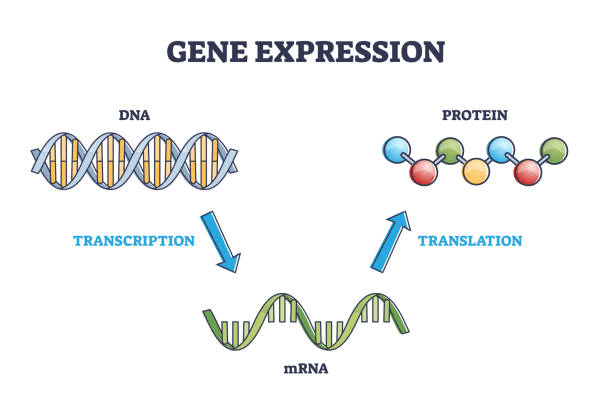
Which of the following correctly orders the steps of gene expression? (First --> Last)
a. mRNA, Transcription, DNA, Translation, Protein
b. Protein, Translation, mRNA, Transcription, DNA
c. DNA, Transcription, mRNA, Translation, Protein
d. DNA, Translation, mRNA, Transcription, Protein
c. DNA, Transcription, mRNA, Translation, Protein
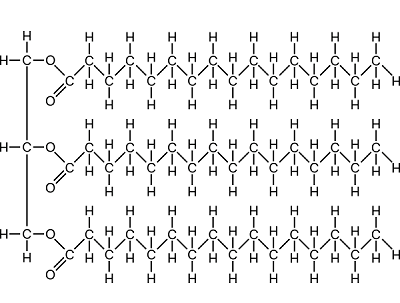
You find a bacterium growing in pure oil, containing the usual hydrophobic lipids. This organism must be very good at break down _______
a. Polar covalent bonds
b. Hydrogen bonds
c. Ionic Bonds
d. Nonpolar covalent bonds
d. Nonpolar covalent bonds
Hydrophobic
- Does NOT mix well with water
- Contain primarily nonpolar covalent bonds
Water has an unusually high specific heat. What does this mean?
a. At its boiling point, water changes from liquid to vapor.
b. A large amount of heat is required to raise the temperature of water
c. Ice floats in liquid water
d. Salt water freezes at a lower temperature than pure water
b. A large amount of heat is required to raise the temperature of water
Water’s ability to moderate temperatures is important for life on Earth because
• Organisms are made primarily of water and able to resist change in their own
temperature.
• Water stabilizes ocean temperatures, creating favorable environment for marine life.
Which biological molecule have hydrocarbon components?
a. Proteins
b. Nucleic Acid
c. Lipids
d. Carbohydrates
c. Lipids
Hydrocarbons are organic molecules consisting of only carbon and hydrogen
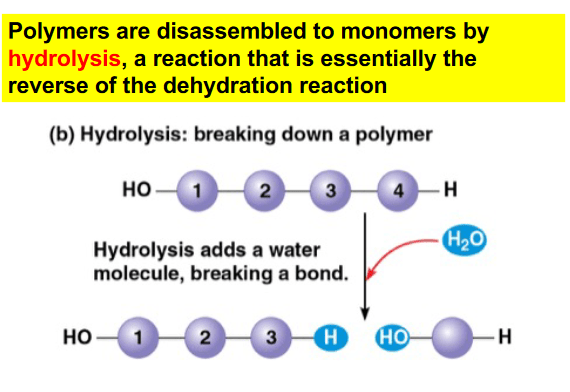
What reaction is depicted below?
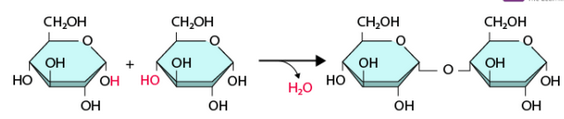
a. Dehydration reaction
b. Hydrolysis
c. Dissolution
d. Oxidation
a. Dehydration reaction
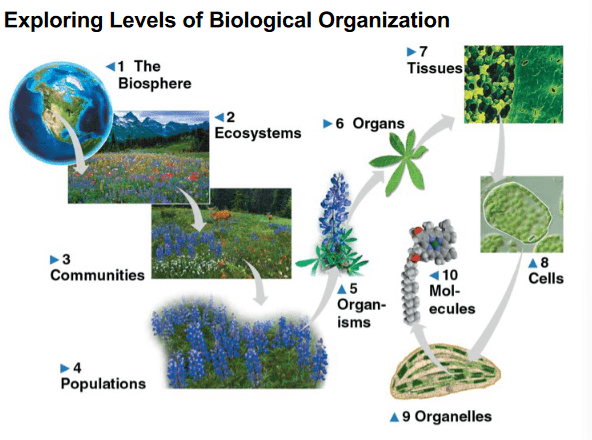
Which of the following correctly orders the levels of organization from most inclusive to least inclusive?
a. Ecosystem, Population, Community, Cell
b. Cell, Tissue, Organism, Community
c. Organism, tissue, cells, molecules
d. Population, Community, organ, organelles
c. Organism, tissue, cells, molecules
How many neutrons does the following isotope have?
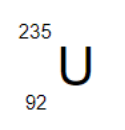
a. 92
b. 235
c. 143
d. 327
c. 143
Mass number: 235
Atomic number: 92
Neutrons= Mass number - Atomic number
235-92= 143
When oil is placed in a cup filled with water, the oil separates from the water forming a layer on top. Which of the following best explains this phenomenon?
a. Oil is comprised of mainly polar covalent bonds and is hydrophilic
b. Oil is hydrophobic because it is consistent of nonpolar hydrocarbon bonds.
c. Oil is hydrophilic because of its ionic nature.
d. Water does not mix well with oil because water has nonpolar covalent bonds while oil has polar covalent.
b. Oil is hydrophobic because it is consistent of nonpolar hydrocarbon bonds.
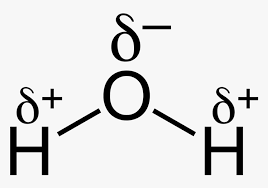
Given the following Lewis dot structures, which answer choice correctly identifies the valence of each atom?

a. H=1, O=3, N= 5, C=6
b. H=1, O=8, N=4, C=6
c. H=1, O= 2, N=3, C=4
d. H=2, O=3, N=5, C=4
c. H=1, O= 2, N=3, C=4
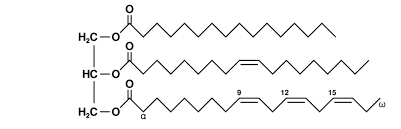
All of the following correctly pairs the biological molecule with its monomer EXCEPT:
a. Proteins are composed of amino acids
b. Lipids are composed of fatty acids
c. Carbohydrates are composed of monosaccharides
d. Nucleic acids are composed of nucleotides
b. Lipids are composed of fatty acids
In this figure, what is the dependent variable?
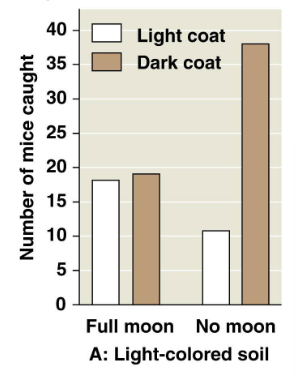
a. The presence or absence of moonlight
b. The mouse coat color
c. The number of mice caught
d. The color of the soil
C. The number of mice caught
Quick Vocab
- Hypothesis: an explanation, based on observations and assumptions, that lead to a testable prediction.
- Independent variable: Changed or controlled by the experimenter
- Dependent variable: The variable being measured in response to the other variables.
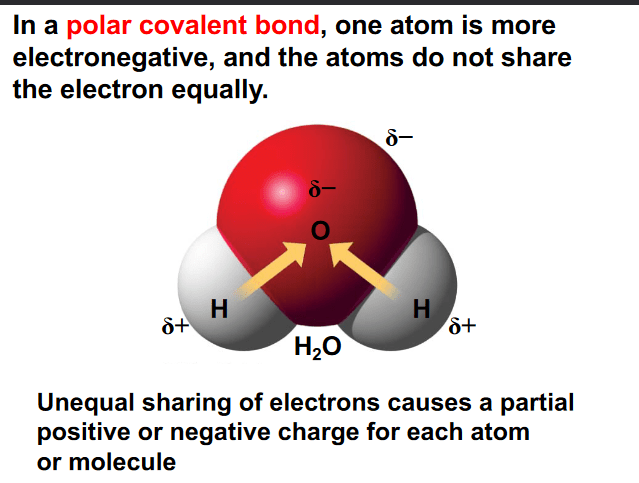
All of the following correctly describe the structure of water EXCEPT:
a. Water contains polar covalent bonds because of a large difference in electronegativity between oxygen and hydrogen
b. The hydrogen atoms contain partial positive charges
c. The oxygen atom contains a partial negative charge
d. There is a equal sharing of electrons between oxygen and hydrogen
d. There is a equal sharing of electrons between oxygen and hydrogen
Which of the following correctly identifies the charge of the green and yellow ions?
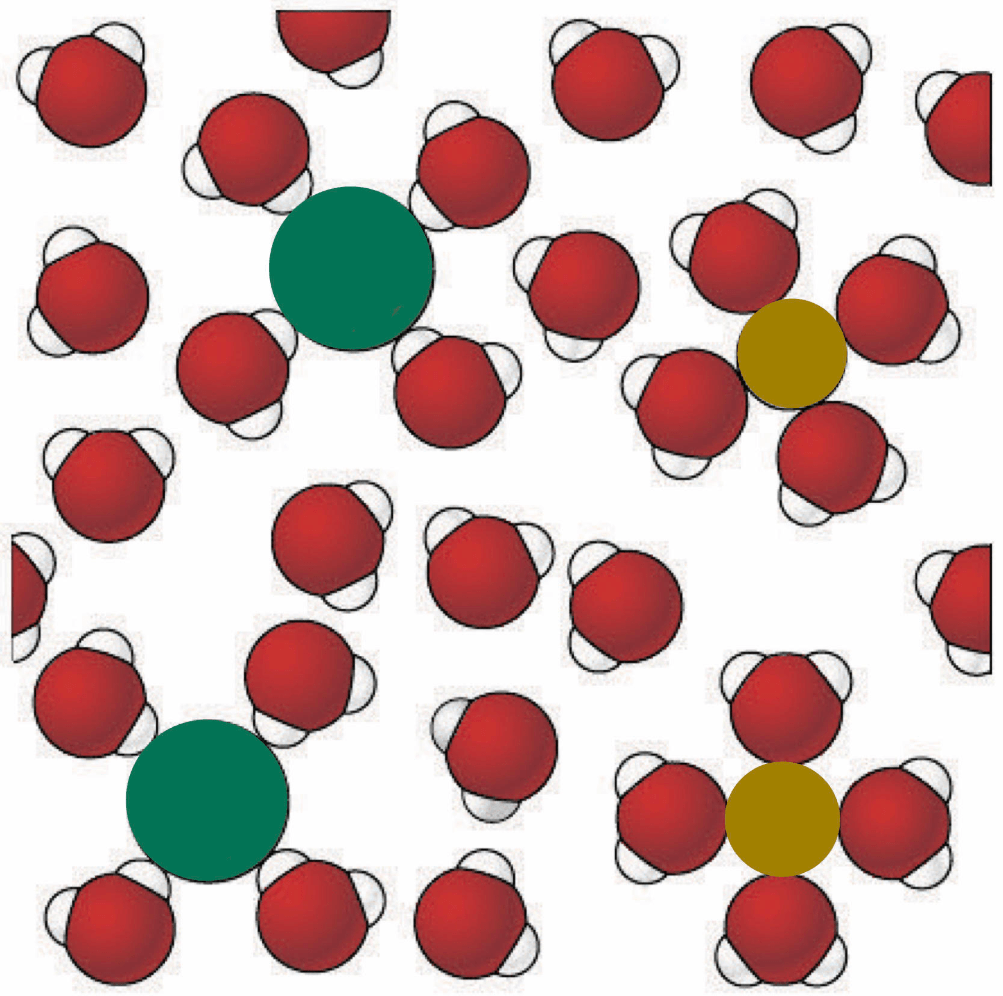
a. Green (+), Yellow (-)
b. Green (-), Yellow (+)
c. Yellow (-), Green (-)
d. Yellow (+), Green (+)
b. Green (-), Yellow (+)
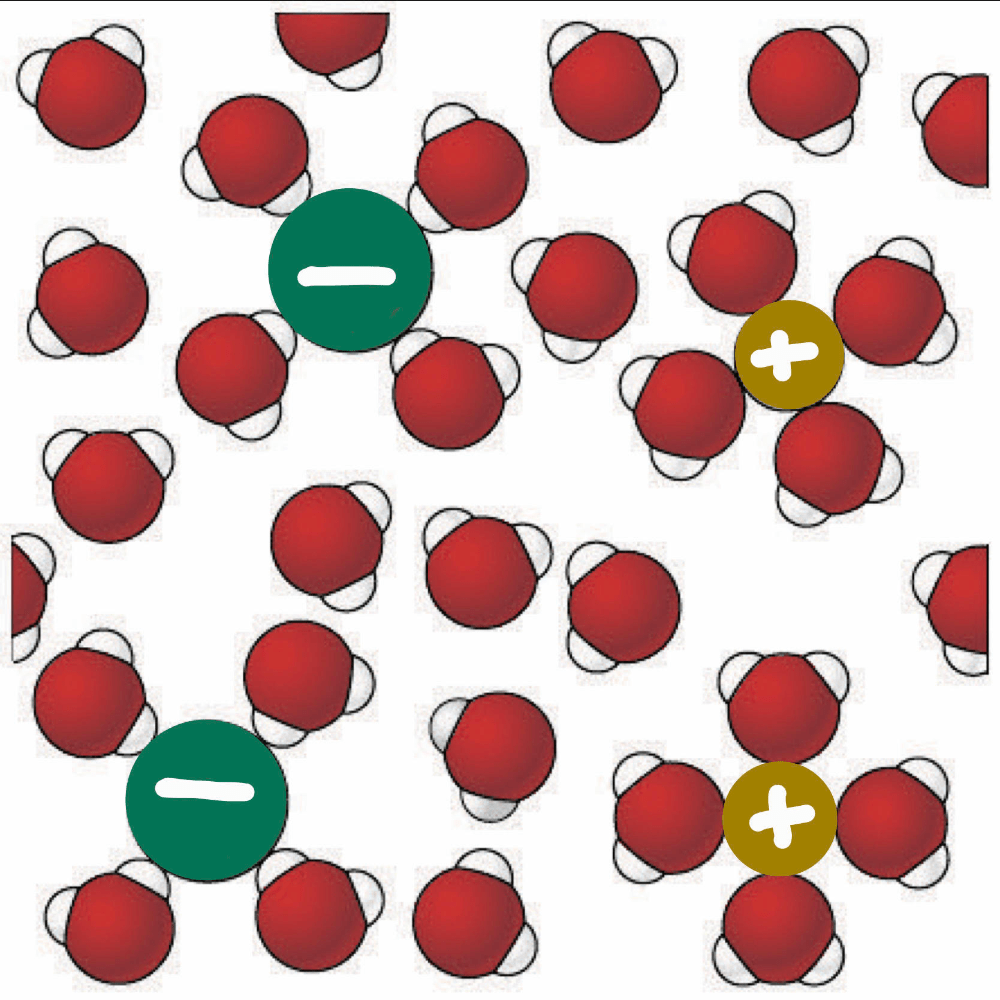
What is the relationship between the following molecules?
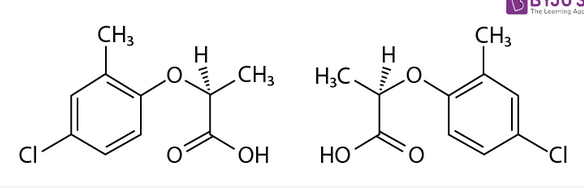
a. Enantiomers
b. Cis-trans isomers
c. Structural isomers
d. Same molecule
a. Enantiomers
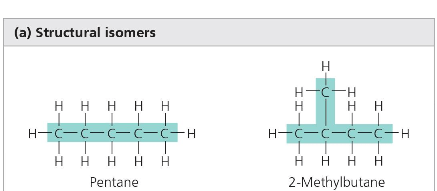
Differ in the arrangement of covalent bonding partners.
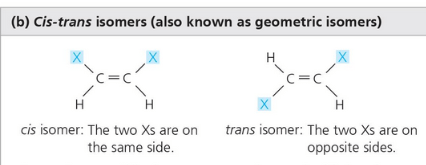
Cis-trans differs in arrangement about a double bond
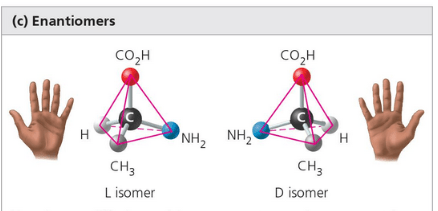
Differ in spatial arrangement around an asymmetric carbon, resulting in mirror images
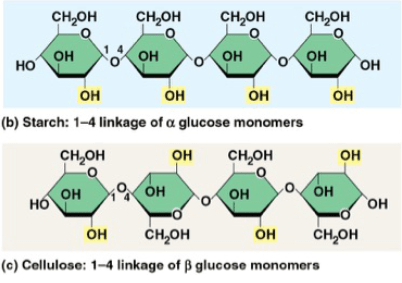
Which carbohydrate can humans digest?
I 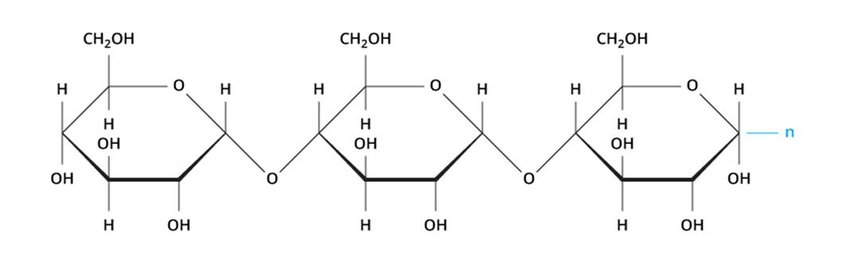 II
II 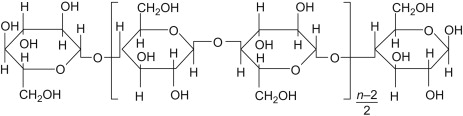
a. Humans can digest carbohydrate I because humans contain enzymes that hydrolyze alpha glycosidic bonds
b.Humans can digest carbohydrate I because humans contain enzymes that hydrolyze beta glycosidic bonds
c. Humans can digest carbohydrate II because humans contain enzymes that hydrolyze alpha glycosidic bonds
d. Humans can digest carbohydrate II because humans contain enzymes that hydrolyze beta glycosidic bonds
a. Humans can digest carbohydrate I because humans contain enzymes that hydrolyze alpha glycosidic bonds
All of the following are observations Darwin made, except:
a. Individuals in a population vary in their traits, many of which seem to be heritable
b. Species are generally well-suited to their environment
c. More offspring are produced than survive, and competition is inevitable
d. Individuals that are best suited to their environment are more likely to survive and reproduce
d. Individuals that are best suited to their environment are more likely to survive and reproduce

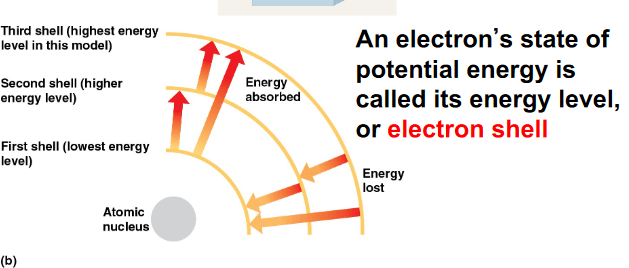
During photosynthesis when light strikes the PSI complex, chlorophyll absorbs the photon resulting in an electron becoming excited (higher energy state). Which of the best describes the electron in the excited state?
a. The excited electron moved to a lower electron shell
b. The electron released energy to reach the excited state
c. The excited electron moved further away from the nucleus
d. The electron absorbed energy and did not move to a different electron shell
c. The excited electron moved further away from the nucleus
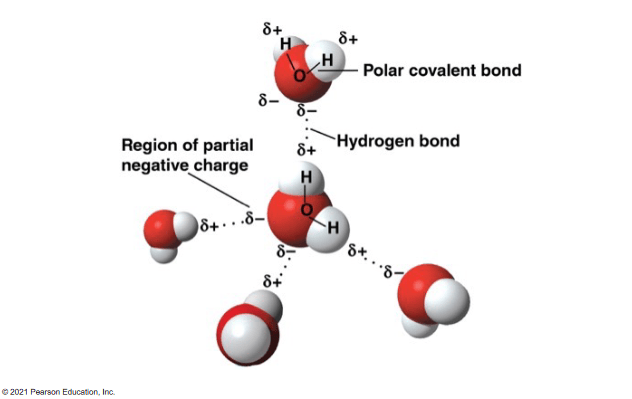
All of the following correctly describes two water molecules hydrogen bonding EXCEPT:
a. The oxygen atom on one molecule shares electrons with a hydrogen atom on the other molecule.
b. There is an electrical attraction between the hydrogen atom on one molecule with the oxygen on the other.
c. The two water molecules are bonded together via an intermolecular force
d. Water molecules can hydrogen bond because each water molecule contains polar covalent bonds
a. The oxygen atom on one molecule shares electrons with a hydrogen atom on the other molecule.
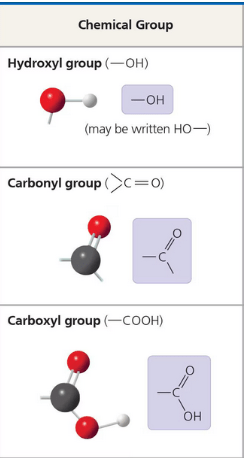
Tyrosine contains all of the following functional groups EXCEPT:
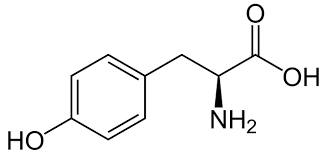
a. Hydroxyl
b. Amino
c. Carboxyl
d. Methyl
d. Methyl
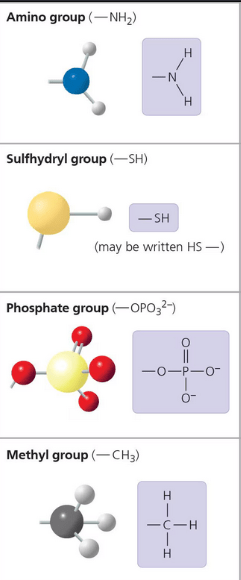
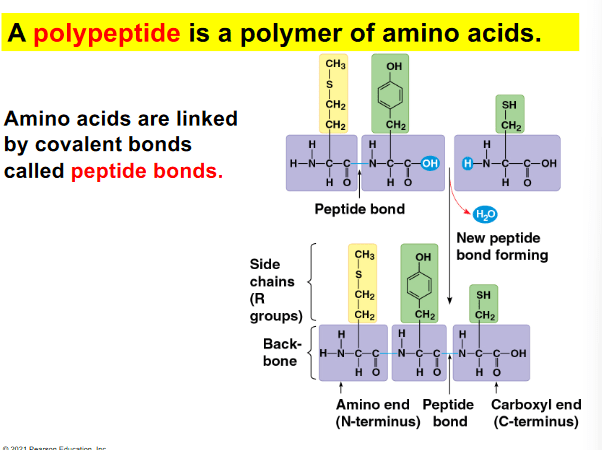
Which of the following statements based describes the bonding of two amino acids?
a. The carboxyl group of the first amino acid will form a peptide bond with the amino group on the second amino acid, via a dehydration reaction.
b. The amino group of the first amino acid will form a peptide bond with the carboxyl group on the second amino acid, via a dehydration reaction.
c. The carboxyl group of the first amino acid will form a peptide bond with the amino group on the second amino acid, via a hydrolysis reaction.
d. The amino group of the first amino acid will form a peptide bond with the carboxyl group on the second amino acid, via a dehydration reaction.
a. The carboxyl group of the first amino acid will form a peptide bond with the amino group on the second amino acid, via a dehydration reaction
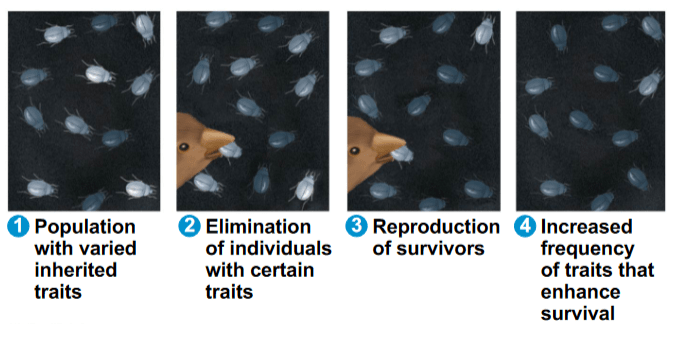
After exposing a population of bacteria to penicillin (an antibiotic), within six months the bacteria are resistant to penicillin. Which of the following best explains how the bacteria evolved?
a. In response to the change in the environment, individual bacteria developed genes that gave resistance.
b. Natural selection created individuals who are resistant to penicillin, a favorable trait
c. Individuals acquired resistance during their lifetime and passed down the resistance to penicillin to their offspring.
d. Natural selected acted on individuals already present with resistant genes, increasing their survivorship and reproduction
d. Natural selected acted on individuals already present with resistant genes, increasing their survivorship and reproduction
Key Things to Remember about Evolution
- Traits acquired during an individual's lifetime do NOT get passed down to their offspring.
- Natural selection does NOT create individuals with favorable traits, but rather acts upon individuals who are present.
Summary of Evolution
1. Individuals within a population vary in their heritable traits (genetic information)
2. Change in environment occurs
3. Natural selection acts upon individuals present who have heritable traits well-suited for their particular environment
4. There is an unequal survival and reproduction amongst individuals in a population.
- Individuals with favorable traits will survive longer and reproduce more.
5. Final result is an increase in the favorable trait within the population over time
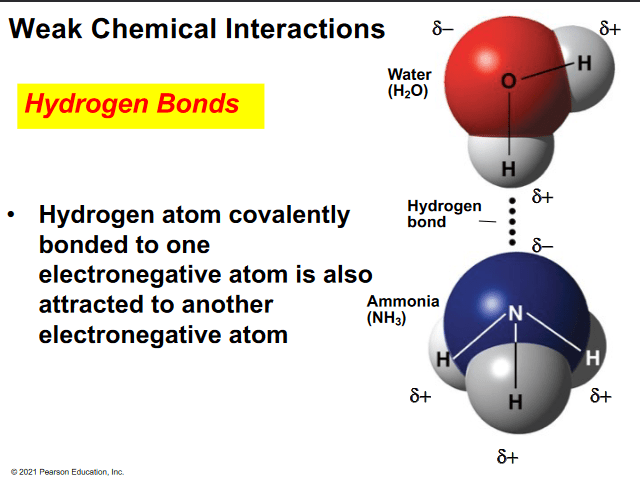
Can ammonia hydrogen bond with water?
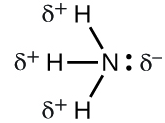
a. No because ammonia contains only nonpolar covalent bonds
b. yes, the hydrogen atom on water will hydrogen bond with the nitrogen atom on ammonia
c. No, because ammonia contains only ionic bonds
d. yes, the oxygen atom on water will hydrogen bond with the nitrogen atom on ammonia
b. yes, the hydrogen atom on water will hydrogen bond with the nitrogen atom on ammonia
Compared to a basic solution at pH 9, an acidic solution at pH 6 has
a. 1000 times more hydroxide ions
b. 1000 times fewer hydroxide ions
c. 3 times more hydrogen ions
d. 3 times fewer hydrogen ions
b. 1000 times fewer hydroxide ions
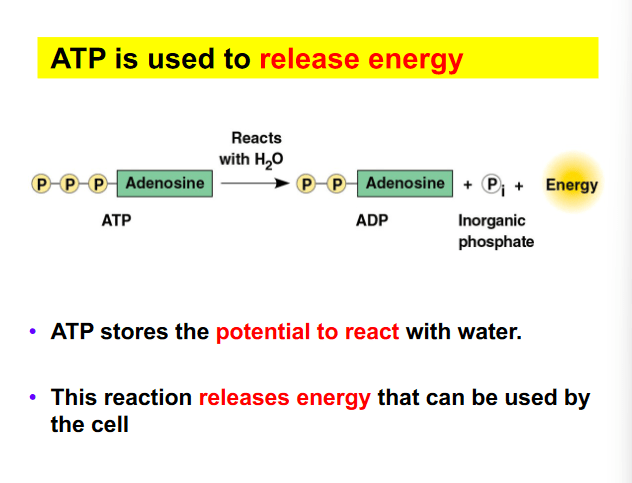
Which of the following correctly identifies ATP and its function?
I
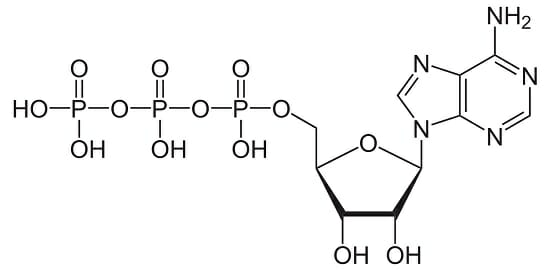
II
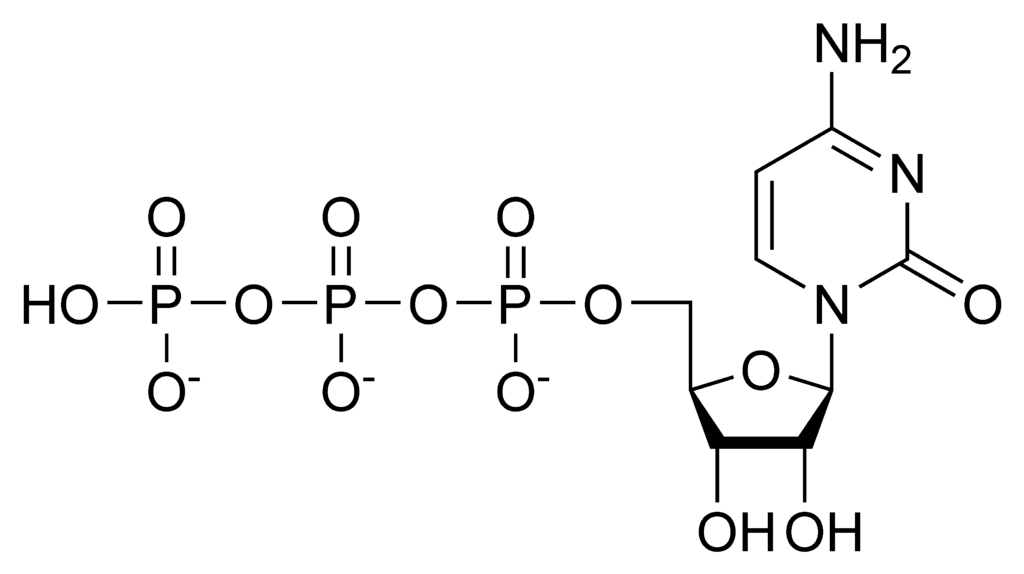
III
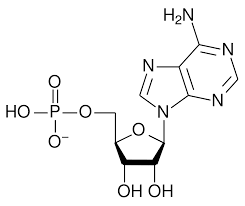
a. I, important source of energy to power cellular processes
b. II, store and transmit genetic information
c. III, fuel storage and building material
d. III, releases large amount of energy when reacted with water
a. I, important source of energy to power cellular processes
A DNA molecules with 15% thymine bases would have what percentage of cytosine?
a. 15%
b. 70%
c. 35%
d. 28%
c. 35%
A+T= 30%
C+G=70%
Since Cytosine and Guanine always pair, there will be an equal number of both. Thus 70%/2= 35%