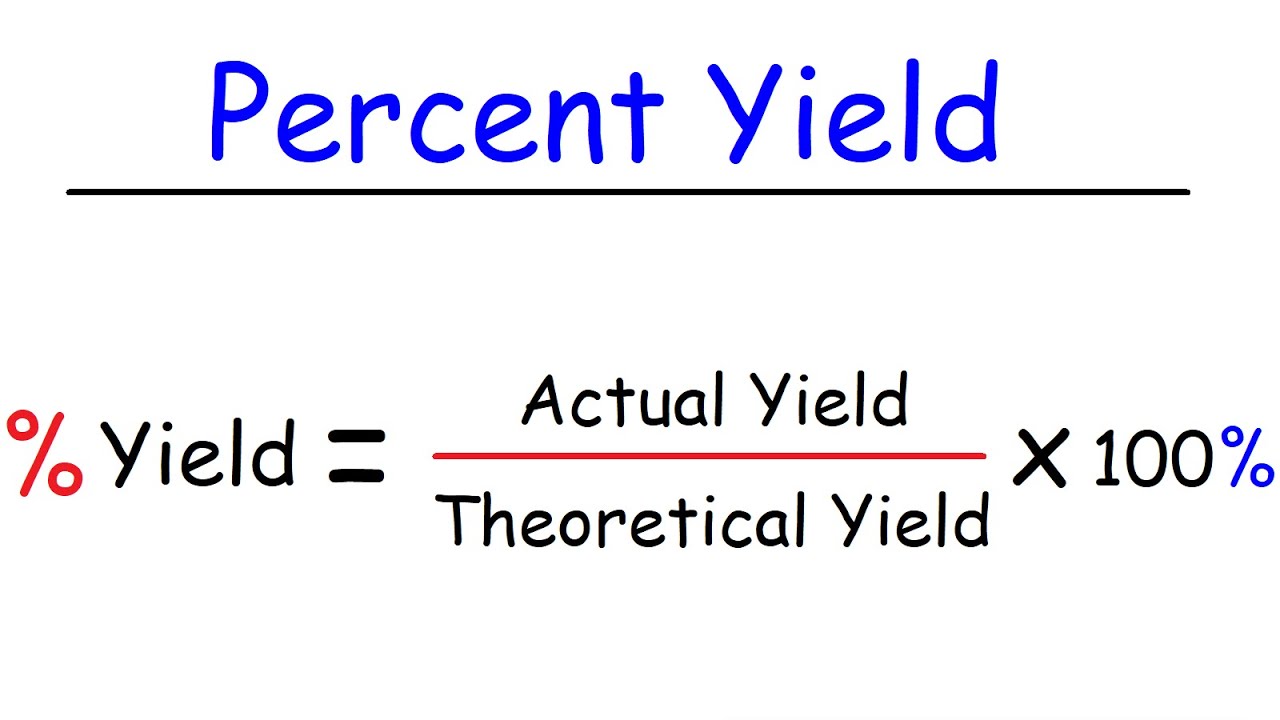
What is the formula for percent yield?
What is the definition of a limiting reactant? An excess reactant?
LR: the reactant that gets consumed first in a chemical reaction and therefore limits how much product can be formed
ER: a reactant in a chemical reaction that is present in a greater quantity than is needed to fully react with the limiting reactant
Define Empirical and Molecular formula.
Which is a longer formula? Shorter?
Empirical: a chemical formula showing the simplest ratio of elements in a compound
Molecular: The true number of molecules in the formula
What is the molarity formula?
What is the dilution formula?
(WITH UNITS)
M1V1=M2V2

What was the Netflix show that is based on financial struggles being solved through childhood games-gone deadly?
Squid Games
In an experiment, 30g of CH4 was produced, although based on my calculations we should have produced 60g. What is out percent yield?
50%
You are given a certain amount of CH4 and O2. The amount of product produced from CH4 is 10g. The amount of product produced from O2 is 5g.
What is the limiting and excess reactants?
LR: O2
ER: CH4
Triethylenemelamine has an empirical formula of C3H4N2 and a molar mass of 204.23 g/mole. What is the correct molecular formula?
C9H12N6
How many liters are needed to make a 5M solution with 2 moles of HCl?
0.4L
What was the show that came out in 2020 where teenagers had to hunt for treasure in North Carolina?
Outer Banks
For the equation shown below, if the reaction of 99.8 grams of O2 produces 51.0 grams of CO2, what is the percent yield?
4C2H5Cl + 13O2 → 8CO2 + 10H2O + 2Cl2
60.7%
What is the limiting reagent if 78 grams of Na2O2 were reacted with 29.4 grams of H2O?
2Na2O2 + 2H2O → 4NaOH + 3O2
LR: Na2O2
What is the correct empirical formula for eplerenone, C24H30O6?
C4H5O
How many grams of NaBr (molar mass = 102.9 g/mol) would be needed to prepare 700mL of 0.230 M NaBr solution?
16.5669gNaBr
What is the spanish crime thriller follows a criminal mastermind known as "The Professor"?
Money Heist
For the UNbalanced equation shown below, if the reaction of 33.3 grams of O2 produces 21.6 grams of CO2, what is the percent yield?
C7H16 + O2 → CO2 + H2O
74.2%
24.5 grams of Co is reacted with 2.58 grams of O2. How much excess reactant is left over after the reaction?
Co + O2 → Co2O3
18.21g excess Cobalt
What is the empirical formula for sucralose?
The percent composition is 36.25% C, 4.82% H, 26.75% Cl and 32.19% O.
C12H19Cl3O8
Hydrochloric acid is sold at the store in a 10 M concentration in a 5000mL bottle. How much water will you need to add to make a 3M solution?
16,670mL
Who are the 3 actors that played Spiderman in Spiderman No Way Home?
Tom Holland, Andrew Garfield, Tobey Maguire
1.3g of Oxygen reacts with excess ammonia to produce 0.68g of water. What is the percent yield of this reaction based on the following UNbalanced formula?
NH3 + O2 --> NO + H2O
77%
I mixed 28g Al and 55g Fe2O3 together. If the actual yield of Fe was 33.61g, determine the percent yield of iron
Al + Fe2O3 --> Fe + Al2O3
87.5%
A sample of a hydrocarbon was combusted producing 1.054 g of CO2 and 0.2168 g of H2O. What is the empirical formula for the hydrocarbon?
CH
You have a 50mL solution of ethanol (C2H6O), which has a density of 0.789g/mL. What is the concentration of a solution using all this ethanol and adding 150mL of water?
5.72M
Name 8 Disney Princesses.
Snow White, Cinderella, Aurora, Ariel, Belle, Jasmine, Pocahontas, Mulan, Tiana, Rapunzel, Merida, Moana, Elsa, and Anna.