DOUBLE POINTS
Which of the following molecules can simply diffuse across the plasma membrane (No transport protein required)? Select all that apply
a. H2O
b. O2
c. CO2
d. Na+
a. H2O
b. O2
c. CO2

- The plasma membrane is a mainly hydrophobic barrier. Thus, hydrophobic (nonpolar) molecules can cross the membrane without the need of a transport protein.
DOUBLE POINTS
On the graph below, identify what each letter represents
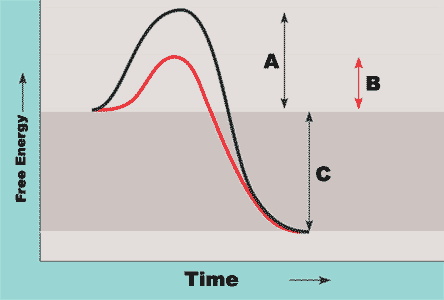
A= Activation energy without enzyme
B= Activation energy with enzyme
C= Change in free energy ∆G
DOUBLE POINTS
Based on the Citric Acid Cycle reaction below, which of the following statements is true?
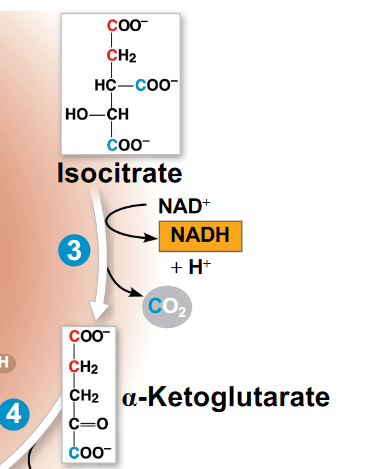
a. Isocitrate is reduced
b. alpha-ketoglutarate is the reducing agent
c. CO2 is the oxidized
d. NAD+ is the oxidizing agent
d. NAD+ is the oxidizing agent
- Isocitrate is oxidized and is the reducing agent
- NAD+ is reduced and is the oxidizing agent
All the following are true regarding photosynthesis EXCEPT
a. The light reactions occur in the thylakoid and the Calvin Cycle occurs in the stroma
b. Water and light are imputed into the light reactions and oxygen, ATP, and NADPH are outputted
c. The source of electrons for photosynthesis come from O2
d. The light reactions and Calvin Cycle occur in the chloroplast
e. The first step of the Calvin Cycle is the fixation of carbon from CO2 onto RuBP
c. The source of electrons for photosynthesis come from O2
Source of electrons is water
DOUBLE POINTS
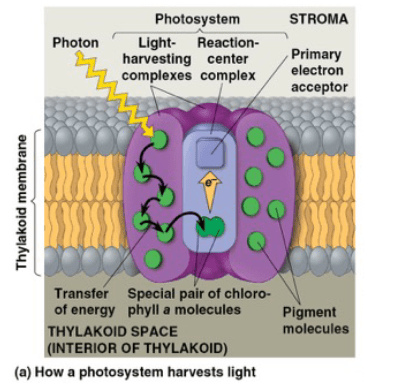
Which of the following statements regarding photosynthesis is true?
a. When a pigment molecule absorbs light, one of its electrons go from the excited state to the ground state.
b. The two products made in the light reactions that are used in the Calvin Cycle are NADH and ATP
c. When light strikes a photosystem, energy is transferred between the pigment molecules in the light harvesting complex and an electron is transferred in the reaction center complex.
d. All of the following are associated with the light reactions: splitting of H2O, releasing of oxygen, oxidation of NADP+, and generation of ATP
c. When light strikes a photosystem, energy is transferred between the pigment molecules in the light harvesting complex and an electron is transferred in the reaction center complex.
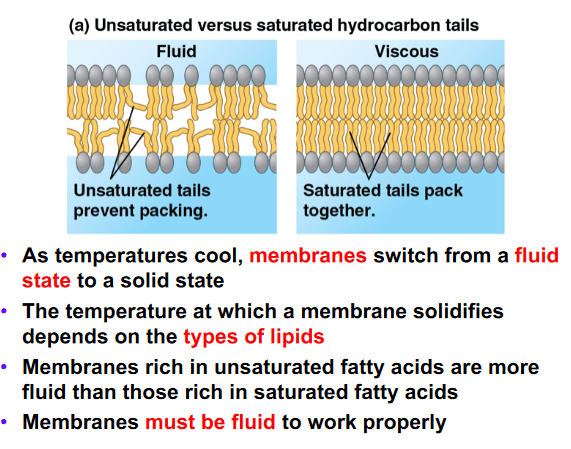
Decreasing the number of unsaturated fatty acid tails will have what effect on the membrane fluidity?
a. The solidifying temperature of the membrane will decrease
b. The membrane will be more fluid at low temperatures
c. The membrane will be less fluid at low temperatures
d. The membrane fluidity will not be significantly effected
c. The membrane will be less fluid at low temperatures
All the following are false regarding enzymes EXCEPT
a. Enzymes are not specific to the substrate they act on
b. Enzymes speed up chemical reactions by lowering the ∆G, change in free energy
c. Enzymes are biological catalysts
d. Enzymatic catalysis occurs in the allosteric site
c. Enzymes are biological catalysts
Enzyme key points
- Act as biological catalyst, they speed up reactions without being consumed in the reaction
- Speed up reactions by lowering the activation energy (do NOT change ∆ G)
- Enzymes are specific to the substrate they bind and catalyze- Catalysis occurs in the active site
- Enzymes work at an optimal temperature and pH range
DOUBLE POINTS
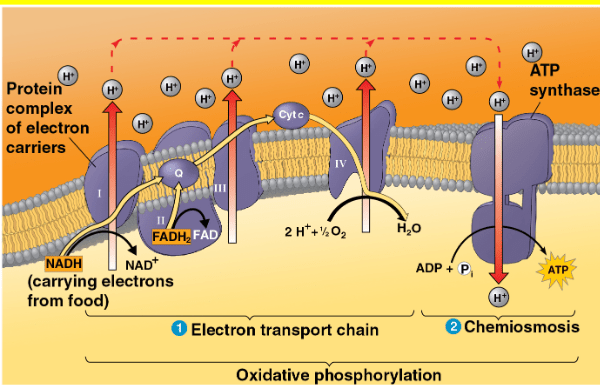
All of the following are true regarding chemiosmosis EXCEPT (select all that apply)
a. Chemiosmosis is powered by the active transport of protons from the inner membrane space to the matrix
b. ATP produced by chemiosmosis is said to be made via oxidative phosphorylation.
c. Chemiosmosis produces ATP in the mitochondrial matrix
d. The proton-motive force couples the electron transport chain to chemiosmosis
e. Complex IV utilizes the proton-motive force established by the ETC to generate ATP via oxidative phosphorylation
A. Chemiosmosis is powered by the active transport of protons from the inner membrane space to the matrix
- passive transport
e. Complex IV utilizes the proton-motive force established by the ETC to generate ATP via oxidative phosphorylation
- ATP synthase
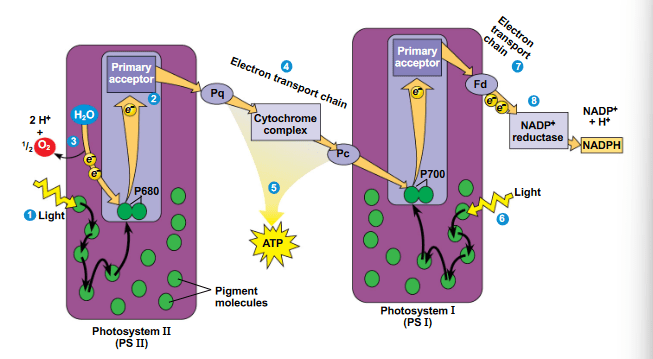
DOUBLE POINTS
Order the following steps of the linear flow of electrons through the light reactions
I Light strikes the light-harvesting complex of PSI and excites the electrons of one of its pigment molecules.
II An enzyme splits water and its released electrons are given to P680+ to restore P680
III Electrons are passed along an electron transport chain eventually reaching NADP+
IV Electrons are passed along an electron transport chain, which uses the released energy to establish a proton gradient
V ATP is generated via chemiosmosis
II, IV, V, I, III
DOUBLE POINTS
In cellular respiration, each NADH leads to the production of 2.5 ATP and each FADH2 leads to the production of 1.5 ATP via oxidative phosphorylation. How much ATP is made by one pyruvate molecule going through stages two and three of cellular respiration?
Stage 2
1. Pyruvate Oxidation
- 1 NADH
- 3 NADH
- 1 FADH2
- 1 ATP
Stage 3: Oxidative Phosphorylation
4 NADH from stage 2= 10 ATP
1 FADH2 from stage 2= 1.5 ATP
Total: 12.5 ATP
Oxidative Phosphorylation: 10+1.5= 11.5 ATPSubstrate Level Phosphorylation: 1 ATP
Which of the following regarding passive transport is false?
a. Facilitated diffusion is a type of passive transport that utilizes transmembrane proteins, such as channels and carriers
b. The passive transport of molecules from very high concentration to very low concentration can power exergonic reactions
c. Water always moves passively from low solute to high solute concentration
d. The movement of molecules from high to low concentration across the plasma membrane without the use of a transporter protein is known as simple diffusion.
b. The passive transport of molecules from very high concentration to very low concentration can power exergonic reactions
- Endergonic reactions (energy consuming)
Passive Transport
- No energy required
- Molecules move from high to low concentration
1. Simple Diffusion
- Passive movement of molecules without the help of a transporter protein
2. Facilitated Diffusion
- Passive movement of molecules with the help of a transporter protein (Channel or carrier)
Which of the following statements is true regarding the diagram below?
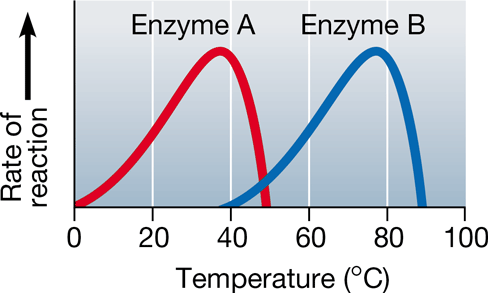
a. Enzyme A works most efficient at temperatures between 0-20 degrees
b. Enzyme B increases the rate of reaction best at temperatures between 85-100 degrees.
c. Enzyme A's primary structure is exposed at temperatures past 50 degrees
d. Enzyme B has decreased catalytic activity as the temperature moves from 60 to 75 degrees.
c. Enzyme A's primary structure is exposed at temperatures past 50 degrees.
- We observe a sharp decline in enzymatic activity when the temperature is too hot because the enzyme is being denatured (3D structure is unfolding)
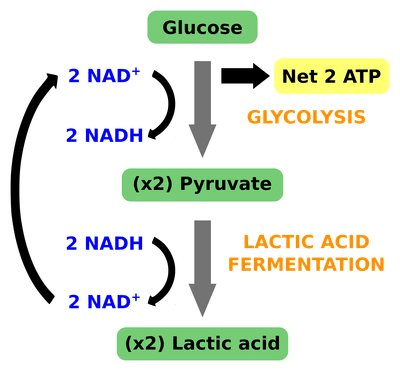
The purpose of lactic acid fermentation is
a. to regenerate glucose by oxidizing pyruvate into lactate
b. to regenerate pyruvate by reducing lactate into acetaldehyde
c. to regenerate NAD+ by oxidizing NADH
d. to regenerate acetyl-CoA by oxidizing pyruvate
c. to regenerate NAD+ by oxidizing NADH
- For glycolysis to continue to occur, there must be a supply of NAD+
- Lactic acid fermentation will regenerate NAD+ by oxidizing NADH
- Thus, allowing glycolysis to continue to occur
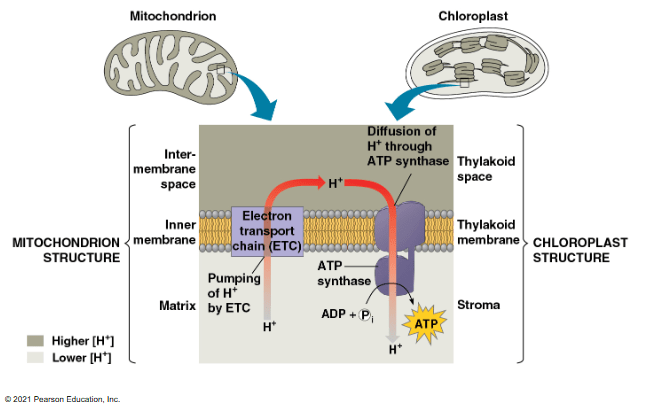
Which of the following statements correctly identifies differences between cellular respiration and photosynthesis?
a. In cellular respiration the electron transport chain is powered by the donating of electrons from NADPH. In photosynthesis, the first ETC is powered by the electrons donated by the primary electron acceptor.
b. In photosynthesis, the first electron transport chain pumps protons into the inter-membrane space. In cellular respiration, the ETC actively moves protons into the thylakoid space.
c. In cellular respiration, ATP synthase is powered by the passive movement of protons from intermembrane space to the matrix. In photosynthesis, ATP synthase is powered by the passive movement of protons from the thylakoid space to the stroma.
d. In photosynthesis, there is a higher concentration of protons in the stroma. In cellular respiration, there is a higher concentration of protons in the intermembrane space.
c. In cellular respiration, ATP synthase is powered by the passive movement of protons from intermembrane space to the matrix. In photosynthesis, ATP synthase is powered by the passive movement of protons from the thylakoid space to the stroma.
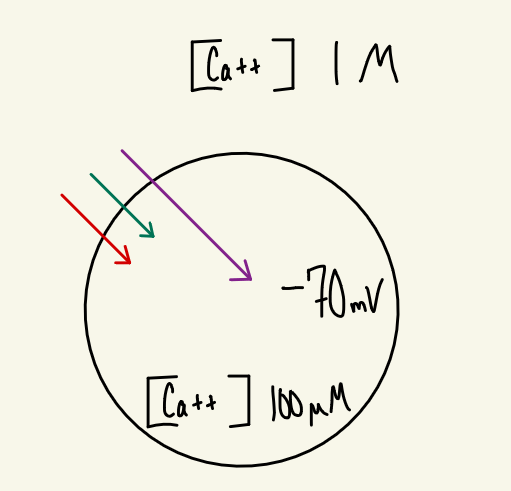
The resting membrane potential of the cell is -70 mV. At resting membrane potential, the extracellular and intracellular concentration of Ca++ is 1 M and 100 µM, respectively. Draw a cell at resting membrane potential and identify the direction of the chemical force, electrical force, and electrochemical force for Ca++.
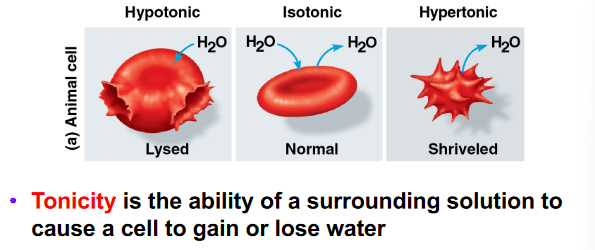
A cell with an intracellular solute concentration of 300 mOsm is placed in a solution with a solute concentration of 250 mOsm. What effect will the solution have on the cell shape?
a. Water will move from low to high solute concentration causing the cell to shrink in size. Thus the solution is hypertonic.
b. The inflow and outflow of water will be equal causing the shape of the cell not to change. Thus, the solution is isotonic.
c. Water will move from low to high solute concentration causing the cell to swell in size. Thus, the solution is hypotonic.
d. Water will move from high to low solute concentration causing the cell to shrink in size. Thus, the solution is hypertonic.
c. Water will move from low to high solute concentration causing the cell to swell in size. Thus, the solution is hypotonic.

Based on the free energy diagram below, which of the following statements are true (Select all that apply)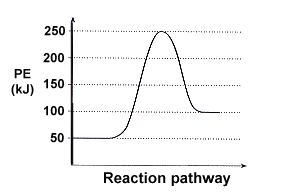
a. The change in free energy (∆ G) is negative because the energy of the products it greater than the reactants
b. The reaction is exergonic and spontaneous
c. The reaction can be powered by coupling it with a highly exergonic reaction
d. Adding an enzyme would decrease Ea and provide the energy needed to power the reaction
e. The reaction releases energy that can be utilized to regenerate ATP from ADP + Pi
c. The reaction can be powered by coupling it with a highly exergonic reaction
Endergonic
- Energy products > energy reactants
- ∆G>0 (Positive)
- Nonspontaneous
- Consumes energy to occur
Exergonic
- Energy products < energy reactants
- ∆G<0 (negative)
- Spontaneous
- Releases energy
All of the following will occur in the absence of oxygen EXCEPT (select all that apply)
a. Pyruvate will be oxidized into Acetyl-CoA and be fed into the Citric Acid Cycle.
b. A net 2 ATP will be produced via substrate level phosphorylation
c. NADH will donate its electron to complex I of the electron transport chain.
d. NADH will reduce pyruvate into lactate.
e. ATP synthase will be powered by the proton-motive force established by the ETC
a. Pyruvate will be oxidized into Acetyl-CoA and be fed into the Citric Acid Cycle.
c. NADH will donate its electron to complex I of the electron transport chain.
e. ATP synthase will be powered by the proton-motive force established by the ETC
- ONLY glycolysis and fermentation can occur in the absence of oxygen
- Thus pyruvate oxidation, The Citric Acid Cycle, and oxidative phosphorylation all need oxygen to occur
All of the following are true regarding linear electron flow EXCEPT (Select all that apply)
a. An electron transport chain is powered to establish a proton gradient
b. The special pair of cholorphyll a molecules called P680 have an electron become excited and donated to a primary electron acceptor
c. ATP synthase is powered by the passive movement of protons from the thylakoid space to the stroma
d. NADP+ reductase reduces NADP+ with the electrons from Fd
e. There is no enzyme catalyzed split of water and release of oxygen
e. There is no enzyme catalyzed split of water and release of oxygen
All the following statements are true regarding the cycle of ATP EXCEPT (select all that apply)
a. The hydrolysis of ATP is a highly exergonic reaction
b. ATP is regenerated from ADP + Pi using the energy supplied by anabolic reactions
c. The hydrolysis of ATP provides the energy to power reactions with ΔG>0
d. Energy needed to regenerate ATP comes from reactions that are non-spontaneous
e. Highly exergonic reactions can supply the necessary energy to add an inorganic phosphate onto ADP.
b. ATP is regenerated from ADP + Pi using the energy supplied by anabolic reactions
- catabolic
d. Energy needed to regenerate ATP comes from reactions that are non-spontaneous
- spontaneous
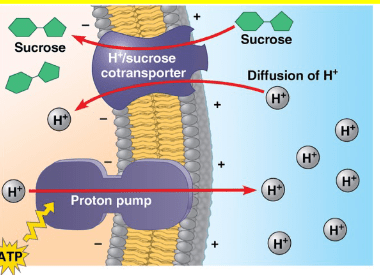
a. The H+/sucrose cotransporter is powered by the active transport of protons into the cytosol.
b. The proton pump utilizes the passive movement of sucrose into the cell to power the active transport of protons out of the cell.
c. The H+/sucrose cotransporter couples the passive movement of protons into the cell with the active transport of sucrose into the cell.
d. If the proton pump were to become inhibited, the transport of sucrose into the cell would increase.
e. The proton pump establishes a steep electrochemical gradient that is utilized by the H+/sucrose cotransporter
a. The H+/sucrose cotransporter is powered by the active transport of protons into the cytosol.
- powered by the passive movement of protons into the cell
b. The proton pump utilizes the passive movement of sucrose into the cell to power the active transport of protons out of the cell.
- The proton pump utilizes the hydrolysis of ATP to actively transport H+ out of the cell
d. If the proton pump were to become inhibited, the transport of sucrose into the cell would increase.
- the transport of sucrose would decrease
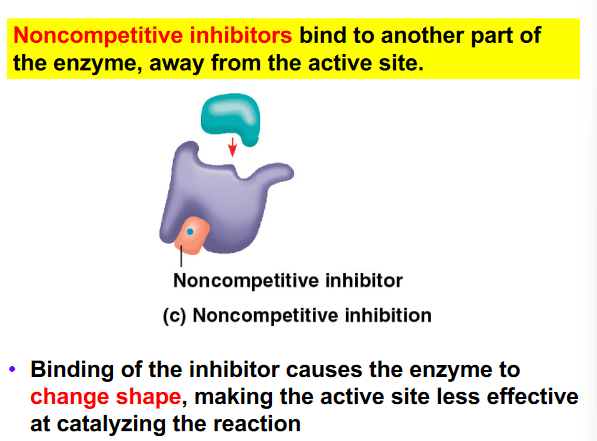
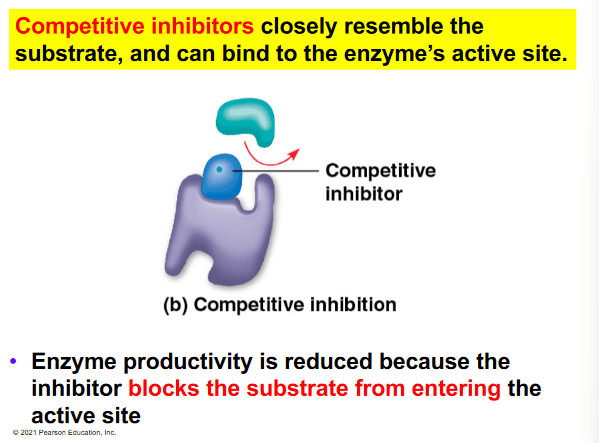
The rate limiting step in glycolysis is the conversion of fructose-6-phosphate (F6P) into fructose bisphosphate (FBP) by the enzyme phosphofructokinase 1 (PFK1). To prevent glycolysis from continuing to occur in the presence of a sufficient amount of ATP, ATP binds to PFK1 at an allosteric binding site, causing the enzyme to have a decreased affinity for F6P. Which of the following statements is true regarding ATP?
a. ATP acts as a competitive inhibitor of PFK1 by binding in place of F6P.
b. ATP acts as both a feedback inhibitor and cofactor of PFK1 by decreasing its catalytic activity.
c. ATP has a similar chemical structure to F6P which allows it to bind to the active site in place of F6P.
d. ATP is a noncompetitive inhibitor PFK1 and is an example of feedback inhibition.
e. ATP acts as a coenzyme for PFK1 resulting in the enzyme having a higher affinity for FBP.
d. ATP is a noncompetitive inhibitor PFK1 and is an example of feedback inhibition.

How many NADH and FADH2 molecules are produced by two molecules of glucose going through cellular respiration?
One glucose molecule
- Glycolysis: 2 NADH
- 2x Pyruvate Oxidation: 2 NADH
- 2x Citric Acid Cycle: 6 NADH, 2 FADH2
= 10 NADH, 2 FADH2
Two glucose molecules
2 (10 NADH, 2 FADH2)= 20 NADH2, 4 FADH2
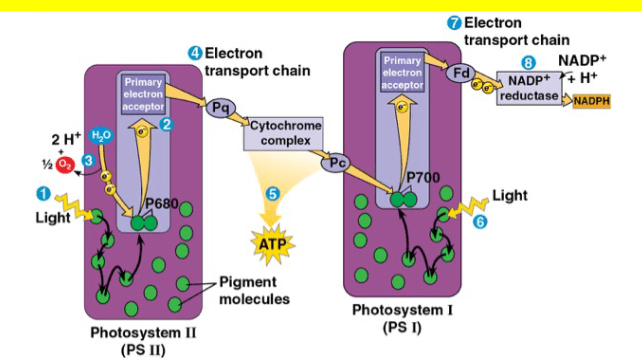
For each statement identify whether it is associated with PSI, PSII, or Both
I Special pair of cholorphyll a molecules are reduced by electrons coming from the splitting of water
II Primary electron acceptor donates electrons to an electron transport chain that pumps protons to establish a proton gradient
III Light strikes the light harvesting complex and energy is transferred between pigment molecules
IV Special pair of cholorphyll a molecules become reduced by electron coming from an ETC
V Energy transferred in the light harvesting complex reaches the reaction center complex causing a pair of cholorphyll a molecules to donate their electron
I Special pair of cholorphyll a molecules are reduced by electrons coming from the splitting of water
- PSII
II Primary electron acceptor donates electrons to an electron transport chain that pumps protons to establish a proton gradient
- PSII
III Light strikes the light harvesting complex and energy is transferred between pigment molecules
- Both
IV Special pair of cholorphyll a molecules become reduced by electron coming from an ETC
- PSI
V Energy transferred in the light harvesting complex reaches the reaction center complex causing a pair of cholorphyll a molecules to donate their electron
- Both
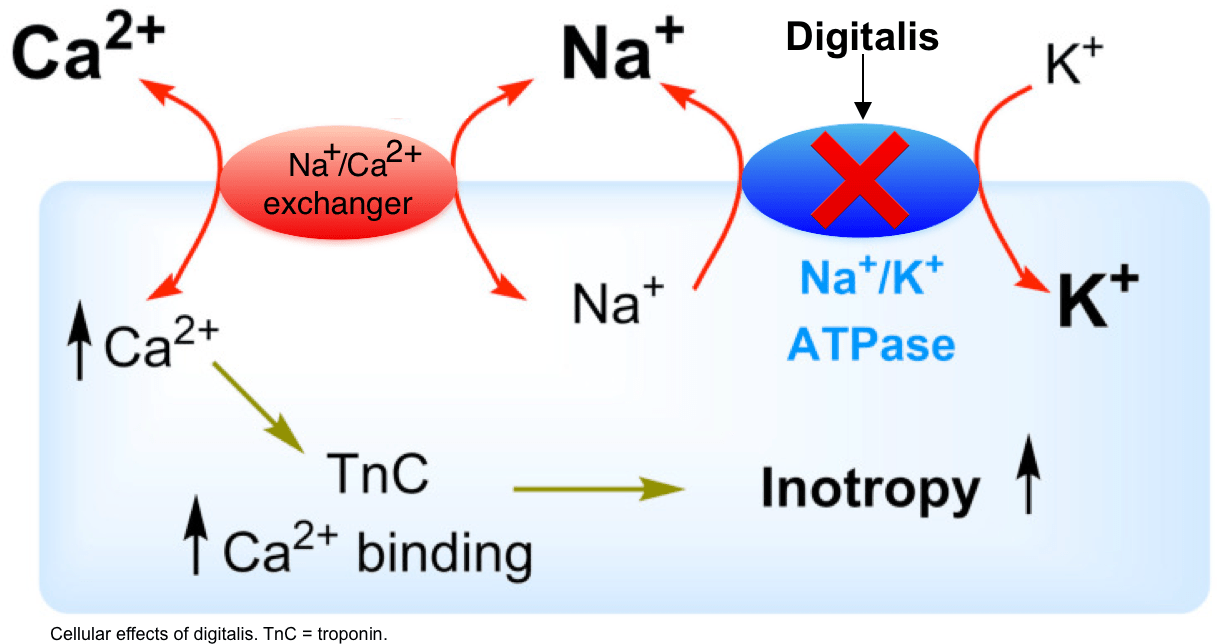
In cardiac muscle cells, the Na+/K+ pump works to maintain a steep concentration gradient of Na+ outside the cell. The Na+/Ca+ exchange pump uses the Na+ gradient established to actively move Ca++ out of the cardiac muscle cells. Quabain is a potent inhibitor of the Na+/K+ pump of cardiac muscle cells. Which of the following statements would be true if an individual was given a dose of quabain?
a. There would be an increase in the extracellular concentration of Na+ resulting in more Ca++ exiting the cell via the exchange pump.
b. The concentration of Ca++ inside the cell would build up because of the dissipation of the gradient that powered the exchange pump.
c. The concentration of K+ outside the cell would decrease resulting in less Ca++ being pumped out of the cardiac muscle cells.
d. The extracellular concentration of Ca++ would increase because the exchange pump would no longer have its source of energy to work.
e. There would be a decrease in the extracellular concentration of Na+ resulting in the exchange pump moving more Ca++ out of the cell.
b. The concentration of Ca++ inside the cell would build up because of the dissipation of the gradient that powered the exchange pump.