Which quantum number distinguishes the sub shell or shape of the orbital?
l
When an electron is attracted to the nuclei of two atoms at the same time
covalent bond
Is it polar?
OCl2
Which type of reaction absorbs energy from the surroundings?
Endothermic
How many valence electrons do Boron and Tin have respectively
3 and 4 respectively
All the possible values of l when n=3.
0, 1, 2
The particle formed when atoms share electrons
What is a molecule
This is the shape of a molecule with three bonds and one lone pair around the central atom
What is Trigonal Pyramidal
A 55 g block of metal has an original temperature of 15.0°C and 0.45 J/g∙°C. This is the final temperature of this metal if 4500 J of heat energy are added.
15.01818 Celsius
Hot packs are an example of this type of reaction.
Exothermic
The total number of electrons in n=4
s-2, p-6, d-10, f-14.
32 total.
The attraction between a positive ion and a negative ion
ionic bond
What is the VSEPR shape for BeCl2
Linear
When S8 and O2 combine to make SO2 2368 kJ are released from the reaction. This is the thermochemical equation for this reaction.
What is S8 + 8 O2 --> 8 SO2 + 2368 kJ
This is the minimum difference in electronegativity required to make a bond polar
0.4
What is the full electron configuration for Copper?
1s22s22p63s23p64s13d10
What element is more electronegative; C or N?
What is N?
What is the VSEPR shape for SF6
Octahedral
A calorimeter’s reaction vessel contains the following reaction:
CuSO4(aq) + 2NaOH(aq) --> Cu(OH)2(s) + Na2SO4(l)
Initially, the water (40.0 mL) in the calorimeter is measured to be 15.0C. After the reaction has taken place, the temperature is 22.0C.
What is the enthalpy of the reaction per mole of CuSO4 if 20.0g of CuSO4 was used in the reaction vessel?
Answer: 1170.4J/0.1253mol = -9.34x103J/mol
The number of bonds in C2H6
What is 7
Which element's highest/last electron has the following quantum numbers: 3, 1, -1, -1/2.
Sulfur
Which of the following properties does lithium have a larger value than potassium; Ionization Energy, Atomic Radius, Electronegativity, or Ionic Radius?
What is ionization energy and electronegativity?
What is the VSEPR shape for NO3-?
Trigonal planar
Complete the Hess's Law Problem below:
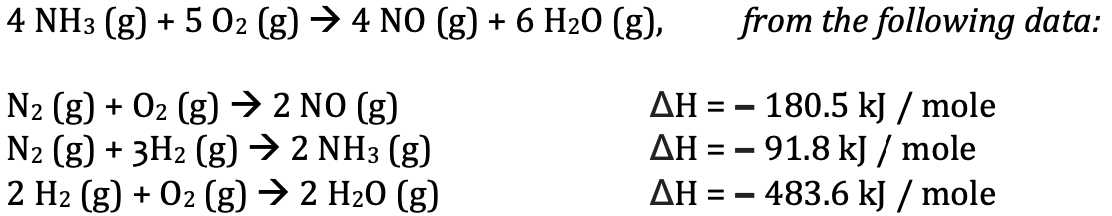
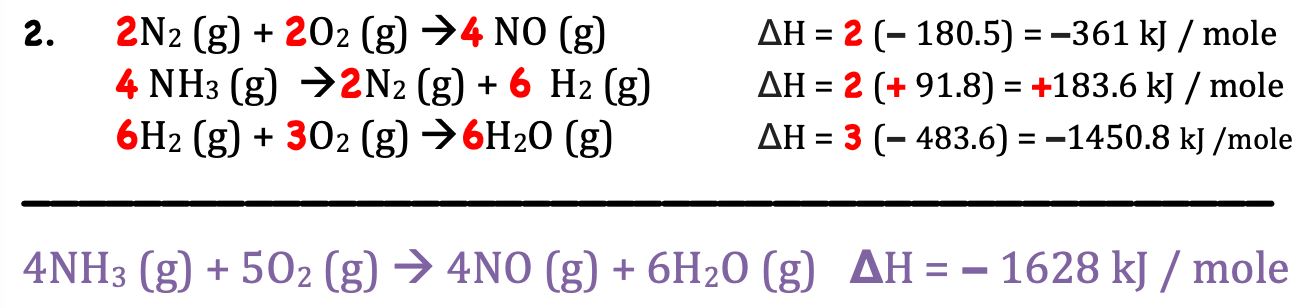
Type of electron pairs that take up the most space
What are lone pairs?