This type of property can be observed without changing the substance.
What is a physical property?
This number tells you how many protons an atom has.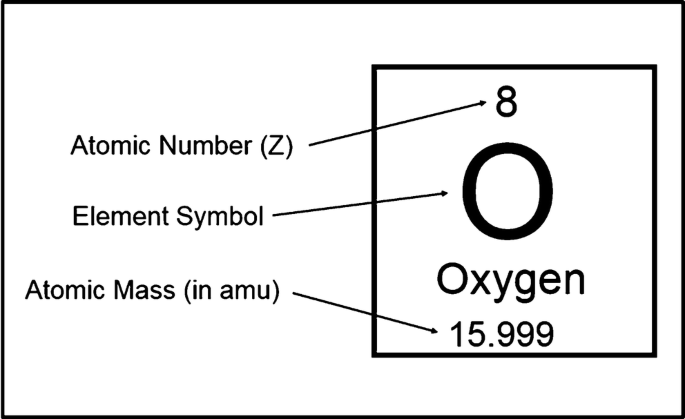
What is the atomic number?
True or false: You can change subscripts to balance an equation.
False
A + B → AB is what type of reaction?
What is synthesis?
What are the 3 options for naming acids?
“hydro-[element]-ic acid” if the compound would end in "-ide"
_____-ic acid if the compound would end in "-ate"
_____-ous acid if the compound would end it "-ite"
Burning paper is this kind of change.
What is a chemical change?
How many electrons does an oxygen atom have if it has 8 protons?
8 electrons
What must be the same on both sides of a balanced equation?
AB → A + B is what type of reaction?
What is decomposition?
How do you name a base?
Name the metal, then say “hydroxide” (e.g., NaOH → sodium hydroxide)?
Flammability is what kind of property?
What is a chemical property?
Carbon has a mass number of 12 and an atomic number of 6, but how many Neutrons?
6 Neutrons
Balance: H₂ + O₂ → H₂O
What is: 2H₂ + O₂ → 2H₂O
AB + C → AC + B is what type of reaction?
What is single displacement?
A base turns phenolphthalein this colour.
What is pink?
Is melting butter a physical or chemical change?
What is a physical change?
Sodium has 11 protons and 12 neutrons. What is its mass number?
23
Balance: Na + Cl₂ → NaCl
What is: 2Na + Cl₂ → 2NaCl?
AB + CD → AD + CB is what type of reaction?
What is double displacement?
Name this acid: H₂SO₄
What is sulfuric acid?
Name 2 signs a chemical change has occurred.
What are: gas, colour change, temperature change, or precipitate?
A chemical element has an atomic number of 19 and a mass number of 39. How many protons, neutrons, and electrons does it have? And what element is it?
Potassium (K)
Balance: Al + O₂ → Al₂O₃
What is: 4Al + 3O₂ → 2Al₂O₃
CH₄ + O₂ → CO₂ + H₂O is this kind of reaction.
Combustion
Name this base: Mg(OH)₂
What is Magnesium Hydroxide?