What tool is used to measure length?
A ruler, tape measure, or meter stick
What state of matter has the lowest energy?
Solid
What element has 16 protons?
Sulfur
In this type of bonding, electrons are STOLEN.
Ionic Bonding
What type of reaction here? How do you know?
2Mg + O2 --> 2MgO
Synthesis
What tool is used to measure volume of a liquid?
A graduated cylinder
How do I turn a solid into a liquid?
Add heat (melt it)
How many total electrons does Chlorine have?
17 electrons
This type of bonding does NOT conduct electricity.
Covalent bonding
What type of reaction here? How do you know?
2Mg + 2HCl --> H2 + 2MgCl
Single displacement
Does this picture show accuracy or precision? 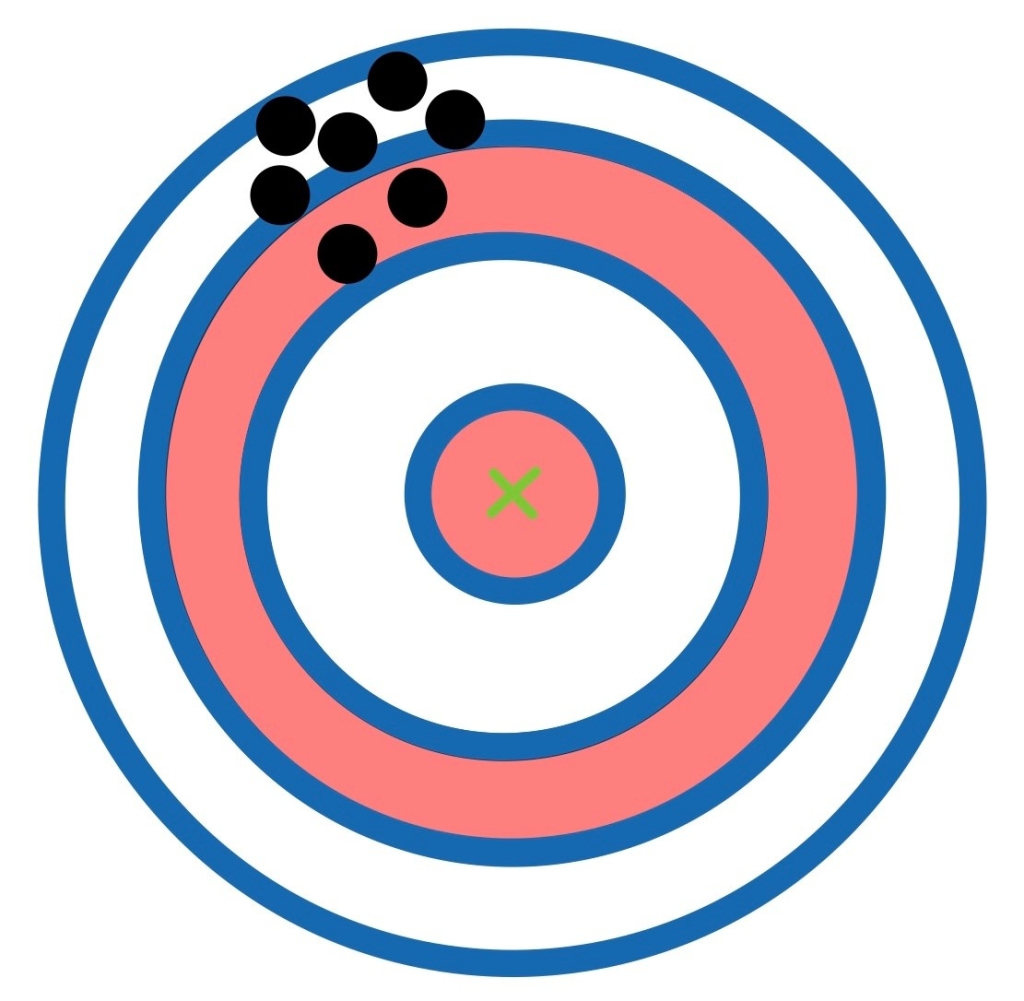
Precision
What state of matter is shown here? 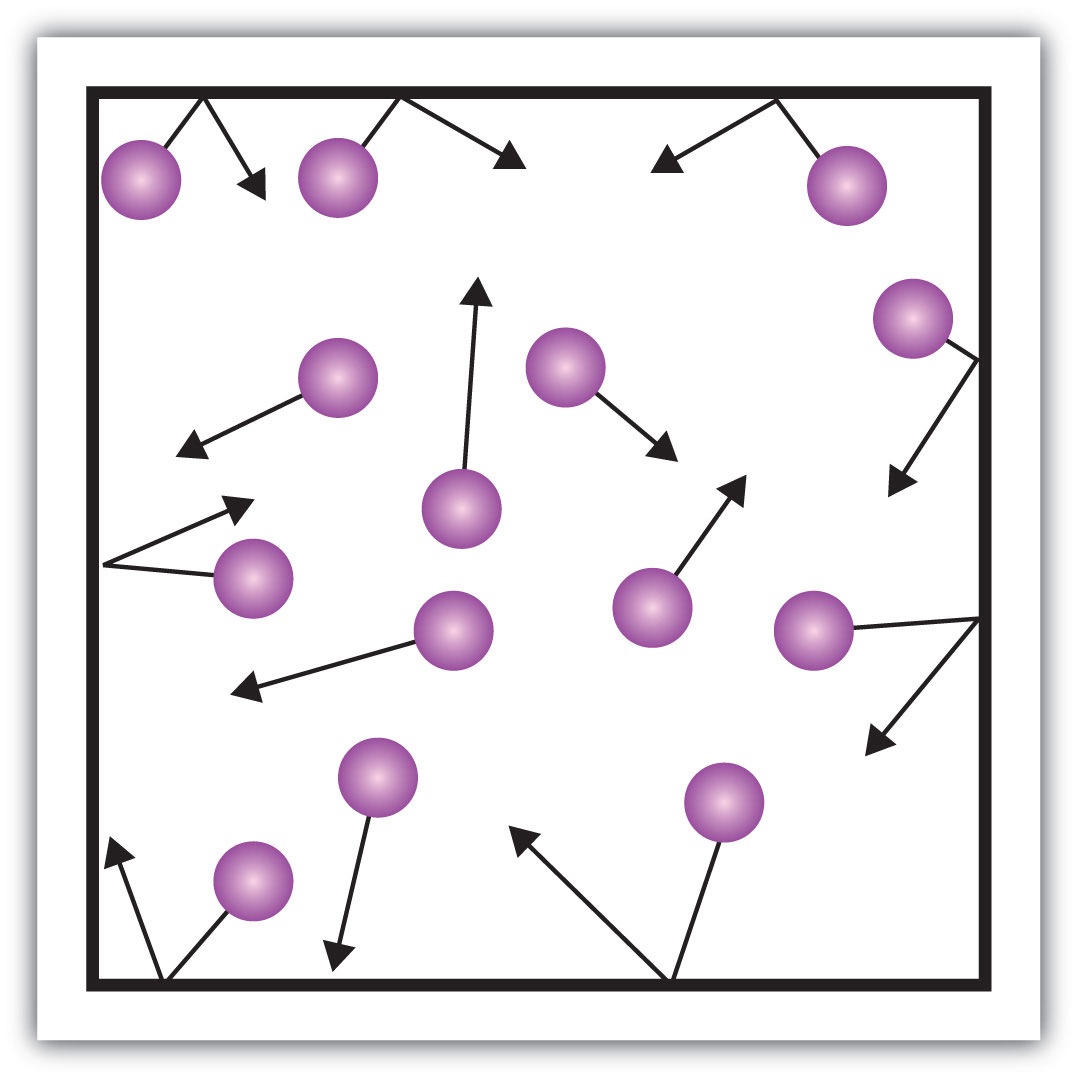
What type of bond would occur between Potassium (K) and Chlorine (Cl)?
Ionic bond
What products do a combustion reaction HAVE to have?
Carbon dioxide (CO2) and water (H2O)
What is the density of an object that has a mass of 10 grams and a volume of 2 mL?
5 g/mL
How do I turn a gas into a liquid?
Remove heat (make it colder)
How many valence electrons does Chlorine have?
7 valence electrons
What would be the final balanced formula for the bonding of Calcium (Ca) and Chlorine (Cl)?
CaCl2
What are 5 signs of a chemical reaction?
Color change, texture change, gas, solid from liquid, change of energy.
What is the mass of an object with a density of 5 g/mL and a volume of 10 mL?
50 grams
What is it called when a gas turns into a liquid?
Condensation
What element is represented here?
Potassium
What would be the final balanced formula for the bonding of Fluorine (F) and Fluorine (F)?
F2
What type of reaction here? How do you know?
AgCl + MgF --> AgCl + MgF
No reaction!!!