The ability for a metal like zinc to react with an acid like hydrochloric acid is classified as this type of property
Chemical Property
These are the five indicators for a chemical reaction
The formation of bubbles
A color change (without a dye)
The formation of a solid or a precipitate
Release of an odor
Temperature change
Silicon-31 contains this many neutrons
17 neutrons
An atom’s ability to attract an electron to itself
Electronegativity
What is the name of the following covalent compound: P4F7
Tetraphosphorous heptafluoride
For the following chemical reactions, balance it and determine the reaction type.
___ Fe + ___ O2 → ____ Fe2O3
4 Fe + 3 O2 → 2 Fe2O3
Synthesis
A material's ability to conduct heat or electricity is classified as this type of property
Physical Property
Model 1 CO2(l) → CO2(g)
Model 2 CO2 (g) → C (s) + O2(g)
Model 2 represents this kind of change
Chemical Change
This is the noble gas configuration of sulfur
[Ne]3s23p4
Half of the distance between the nuclei of 2 atoms that are next to each other
Atomic radius
Write the chemical formula for the following compound:
Iron (III) chloride
FeCl3
For the following chemical reactions, balance it and determine the reaction type.
____ AgNO3 + ____ Fe2(SO3)3 → ____ Ag2SO3 + ____ Fe(NO3)3
6 AgNO3 + 1 Fe2(SO3)3 → 3 Ag2SO3 + 2 Fe(NO3)3
Double Displacement
Double Replacement
A rectangular block of copper metal weighs 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, the density of copper is calculated to be
8.92 g/mL
Model 1 CO2(l) → CO2(g)
Model 2 CO2 (g) → C (s) + O2(g)
Model 1 represents this kind of change
Physical Change
This is the electron configuration for Arsenic
1s22s22p63s23p64s23d104p3
Energy needed to remove a valence electron
Ionization energy
Based on the data, which substance has the strongest intermolecular forces? 
Substance 2
Predict the products and balance the following chemical equation:
___C3H8 + ___ O2 →
C3H8 + 10 O2 → 3CO2 + 4H2O
This volume of silver metal (density of silver = 10.5 g/mL) will weigh exactly 2500.0 g.
238.1 mL
2 Mg (s) + O2 (g) → 2 MgO (s)
The classification for the above reaction
Synthesis or combination
This is why copper appears blue when burned and a diagram that depicts this phenomenon
Because as electrons fall back from an excited state to ground state, they emit light.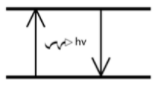
Which element has the highest electronegativity?
A. O B. Ne C. C D. Be
a. Oxygen
Which model represents an ionic compound? Why?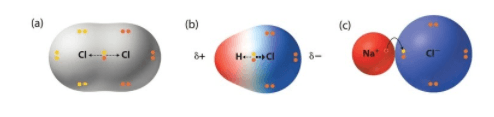
Model C, because there is a transfer of electrons resulting in a cation and anion.
Predict the products and balance the following reaction:
___ Mg + ____ NaI →
1Mg + 2NaI → 1MgI2 + 2Na
The mass of ethyl alcohol (density = 0.789 g/mL) that exactly fills a 200.0 mL container is
157.8 g
Ca(OH)2 (s) + 2 HCl (aq) → CaCl2 (aq) + 2 H2O (l)
The classification for the above reaction
Double-Displacement
Double-Replacement
The average atomic mass for element X is 
28.08 amu
Which element has the smallest atomic radius?
a. Na B. Rb C. Fr D. H
d. Hydrogen
Students in class argue about whether salt (NaCl) or water (H2O) has stronger intermolecular forces. Who does and why?
Salt never melted and water has a lower boiling point, so salt has stronger intermolecular forces.
Predict the products and balance the following chemical reaction:
____ AlN + ____ Ca(OH)2 →
2AlN + 3 Ca(OH)2 → 2 Al(OH)3 + Ca3N2