Write this measurement in scientific notation:
0.00601 m
6.01 x 10-3 m
Is this sample an element or a compound:
an element, palladium
How many sig figs in this measurement?
0.04050 seconds
4, the 4050
Do this calculation and write the answer with the proper amount of sig figs and units:
56.050 g / 87.20 mL
0.6428 g/mL
Which sample is more dense? On the board
Sample A
Which element is in period 6, group 12?
Hg, mercury
How many protons in uranium?
92
Which orbital is this? On the board.
p orbital
Write this measurement in scientific notation:
8,457,100 hours
8.4571 x 106 hours
Is this sample an element or a compound:
a compound (with iron, nickel, cobalt, and sulfur atoms)
Round this measurement to 3 sig figs:
17,295 m
17,300 m
Do this calculation and write the answer with the proper amount of sig figs and units:
43.015 cm - 16.2 cm
26.8 cm
Calculate the density for an object with mass of 5.32 g and volume of 3.25 ml.
1.64 g/mL
Which group is barium in? (special name group)
alkaline earth metals (group 2)
How many electrons in a neutral atom of cadmium?
48
Which orbital requires more energy to be filled with electrons: 3s or 3d?
3d
Write this measurement in regular number format:
9.345 x 108 L
934,500,000 L
Is this sample a mixture or a pure substance:

a mixture
Round this measurement to 4 sig figs:
0.04796532871 g
0.04796 g
Do this calculation and write the answer with the proper amount of sig figs and units:
348.094 g / (187.210 mL - 146.40 mL)
8.530 g/mL
Calculate the density (with proper sig figs and units) for an object with:
mass of 3.4 g
initial water volume: 10.00 mL
final water volume: 13.80 mL
0.89 g/mL
What period and group is bismuth in?
period 6, group 15
How many neutrons in tungsten?
110
Which orbital requires more energy to be filled with electrons: 3d or 5s?
5s
Write this measurement in regular number format:
2.76 x 10-6 g
0.00000276 g
Classify this sample two different ways:
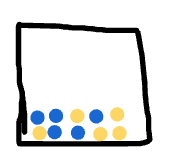
acceptable: mixture of atoms, solid, atoms, different elements
What volume of liquid is in this graduated cylinder? Report your answer with the proper amount of sig figs.
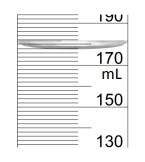
Each line is worth 2 mL, and it looks like it's right on the line before 180 mL so
178.0 mL
Hertz (Hz) = 1/seconds
Convert 341.25 Hz to 1/minute. Write your answer with the proper amount of sig figs.
341.25/s * (60 s)/(1min) = 20475 1/min
Calculate the density (with proper sig figs and units) for an object with:
mass of 23.145 g
initial water volume: 15.00 mL
final water volume: 23.25 mL
2.80 g/mL
What's the halogen in period 2?
fluorine
Which element has 20 neutrons, 20 electrons, and 20 protons?
calcium
How many orbitals total in energy level 3?
9
Write this measurement in scientific notation:
three hundred thousand four hundred minutes
three hundred thousand four hundred = 300,400
3.004 x 105 minutes
Classify this sample two different ways: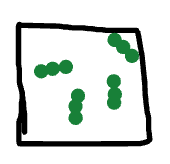
acceptable: element, pure substance, gas, molecule
Report the diameters of the quarter and penny, with the proper sig figs.
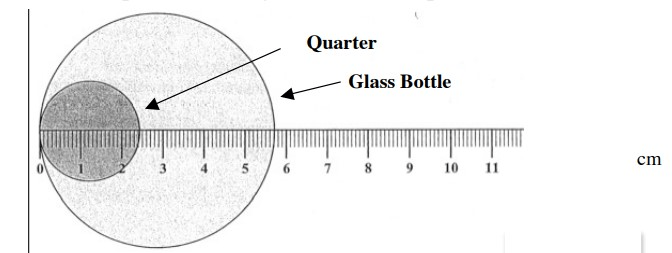
each line is worth 0.1 cm
quarter: 2.40 cm
glass bottle: 5.70 cm
1 mile = 1.60934 km
How many miles are in 127.35 hectometers? Report your answer with the proper amount of sig figs.
100 m = 1 hm and 1000 m = 1 km
127.35 hm * (100 m)/(1 hm) * (1 km)/(1000 m) = 12.735 km
12.735 km * (1 mil)/(1.60934 km) = 7.9132 mil
Which of these elements would react similarly to phosphorous?
a. silicon
b. nitrogen
c. chlorine
d. oxygen
b. nitrogen because they're in the same group (so have the same number of valence electrons)