Classify this sample as a mixture or pure substance.
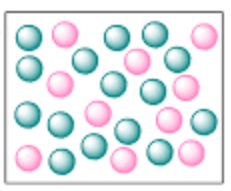
mixture
What is in this sample: atoms, molecules, or atoms and molecules?
atoms
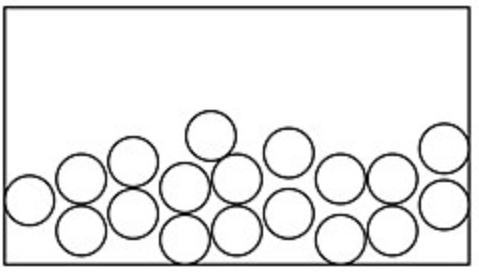
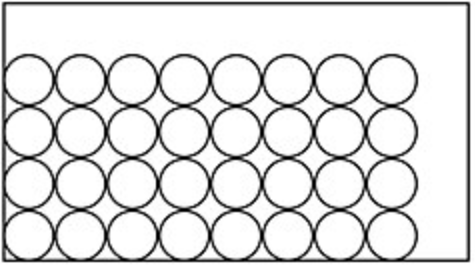
What type of state of matter is in each image?
A
B
A liquid
B solid
50 points for each answer
What's a word that describes these phase changes (aka state of matter change):
a. solid to gas
b. gas to solid
c. liquid to solid
a. sublimation
b. deposition
c. freezing or solidification
25 points per question + 25 points for answering all 3
Which of these is a metalloid?
a. manganese
b. magnesium
c. astatine
e. bromine
c, astatine
Groups are ______, periods are _____.
groups are vertical, periods are horizontal
What are the charges of each subatomic particle?
protons +, electron -, neutrons neutral (zero charge)
What does density measure or quantify?
how compact a substance is, or how many particles are in an amount of volume
What are valence electrons?
The electrons located farthest away from the nucleus
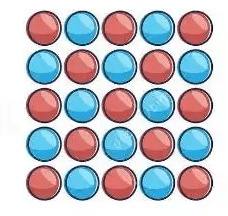
What type of mixture is this sample: heterogeneous or homogeneous?
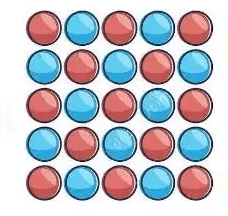
since uniformly mixed, homogeneous
What is in this sample: atoms, molecules, atoms and molecules?

molecules
Which state of matter has the fastest moving particles?
gas
Is this sample being heated or cooled?
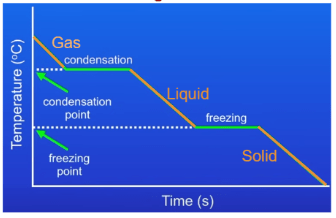
starts at high T at (0,y) and ends up at low T at (x,0) so cooling
Is hydrogen a metal, nonmetal, or metalloid?
nonmetal
What element is in group 4, period 5?
zirconium
How many protons in one atom of iridium?
77
Magnesium has a density of 1.738 g/mL. Would it float or sink in water?
sink, according to the density
In a Bohr model for sulfur, how many electrons in each ring?
1st ring 2 electrons
2nd ring 8 electrons
3rd ring 6 electrons
What type of mixture is this sample: heterogeneous or homogeneous?
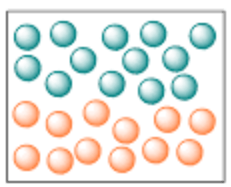
Since there are distinct layers (aka NOT uniformly mixed), heterogeneous
Are the molecules in this sample are an element or a compound?

compound
Which state (or states) of matter can be compressed?
only gases
This is a heating curve for a metal. What is the melting point temperature for this metal?
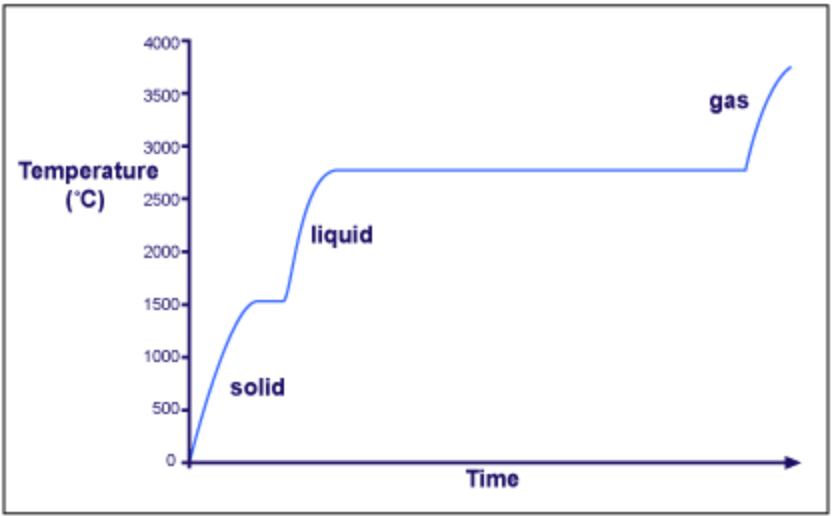
at the first plateau, approximately 1500 degrees C
Which type of material is shiny, flexible, and good at conducting heat and electricity?
a. nonmetals
b. metals
c. metalloids
b, metals
Which of these elements are NOT diatomic? May be more than one answer.
hydrogen, calcium, chlorine, carbon
calcium and carbon
150 points per answer
How many neutrons in one atom of radium?
138
An object has a mass of 65.42 g and a volume of 89.14 mL. What is the density?
density = mass/volume
How many valence electrons in one atom of bismuth?
5
True or false: this sample is a pure substance
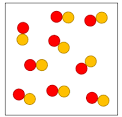
All the molecules in the sample are the same (one red + one yellow), so pure substance
Are the molecules in this sample elements or compounds?
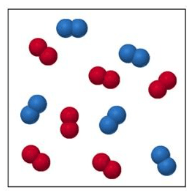
elements
Which state (or states) of matter have a set volume (meaning it's the same volume no matter what container it's in) but a flexible shape?
liquids
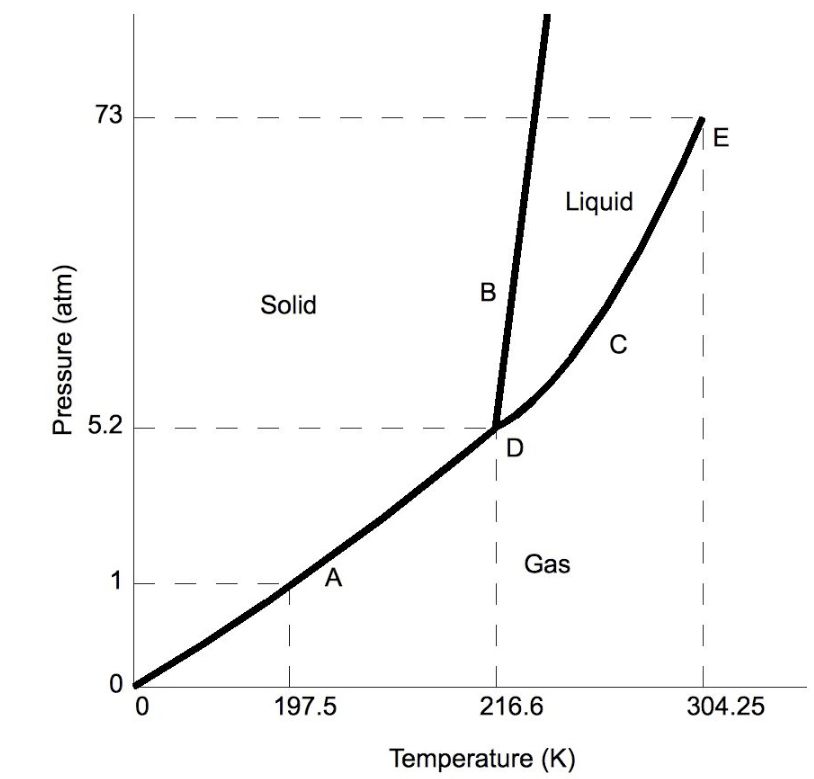
What is the triple point of carbon dioxide?
point D, or 216.6 K and 5.2 atm
Which of these materials would you expect to be brittle, matte (aka not shiny), and a good insulator against heat and electricity?
a. selenium
b. tellurium
c. polonium
d. chromium
a, selenium (nonmetal)
What specialty group is lead in?
post transition metals
What keeps electrons in the atom?
What keeps protons in the atom?
electrons: attraction to protons
protons: the strong force
200 points per question
A rectangular object has a mass of 65.23 grams. It has a length of 12.45 cm, a width of 2.25 cm, and a height of 17.50 mm.
Calculate the density. Hint: change the mm to cm before moving on.
Report answer with correct units and sig figs.
1.23 g/(cm^3)
400 points for all correct
300 points for either sig figs or units wrong
200 points for sig figs and units both wrong
Which neutral atom is shown in this partially redacted Bohr model?
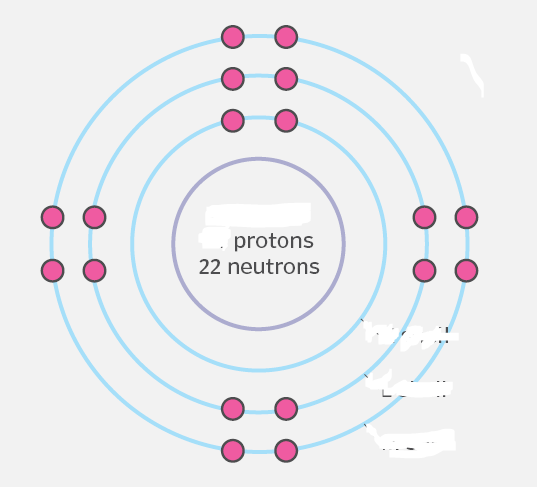
argon
Is this a pure substance or a mixture?

Since it lists one item (sodium bicarbonate) 100%, this is a pure substance
Are these molecules an element or a compound?
a. nitrogen, N2
b. calcium chloride, CaCl2
c. ammonia, NH3
a. element
b. compound
c. compound
all right = 500 points
2 right = 250 points
1 right = 100 points
Which state of matter has the most amount of interactions (NOT bonds) between the particles to keep a set _____ and a set ______?
solids, volume, shape (the last two could be in either order)
A sample of carbon dioxide starts off at 300 K and 1 atm. It is heated up to 305 K and 73 atm. What state of matter describes the carbon dioxide at the second pressure and temperature?
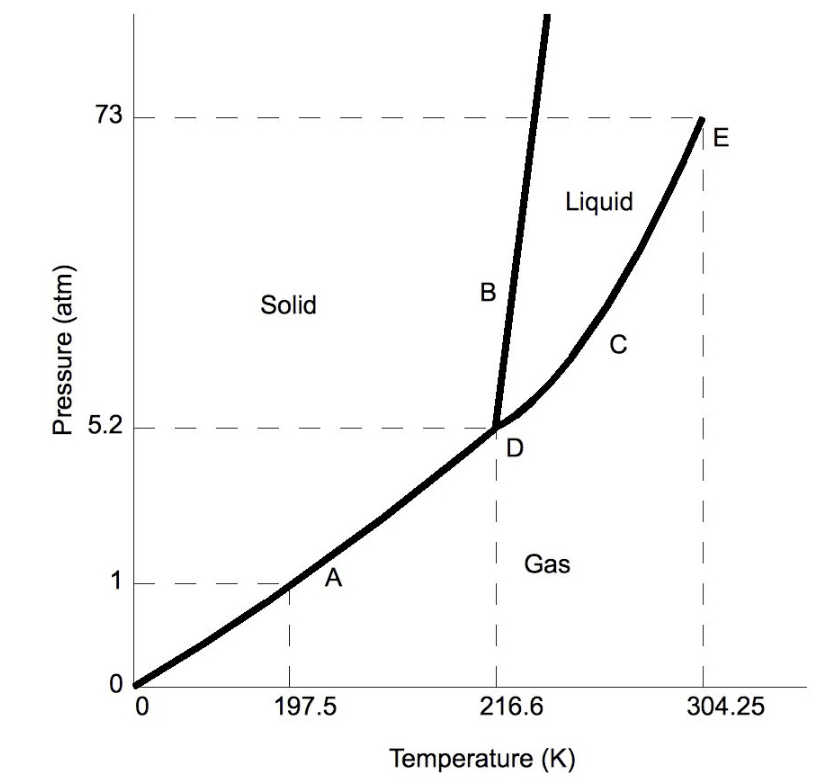
supercritical fluid
Which material may be either shiny or matte, flexible or brittle, and is a semiconductor?
a. mercury
b. titanium
c. germanium
d. iodine
c, germanium
Identify these elements:
a. alkali metal in period 2
b. halogen in period 4
c. last element in the lanthanide row
a. lithium
b. bromine
c. lutetium
3 right = 500 points
2 right = 250 points
1 right = 100 points
How many of each subatomic particles in one neutral atom of:
a. curium
b. tungsten
c. the metalloid in period 2
d. the halogen in period 5
a. 96 protons, 96 electrons, 151 for curium
b. 74 protons, 74 electrons, 10 neutrons
c. boron 5 protons, 5 electrons, 6 neutrons
d. iodine 53 protons, 53 electrons, 74 neutrons
125 points for each answer (all or nothing on the subatomic particle numbers)
Magnesium has a density of 1.738 g/mL.
The volume of a sample of magnesium was measured by water displacement. The initial water volume was 20.00 mL. The final water volume was 28.40 mL.
a. What was the volume of the magnesium sample?
b. What was the mass of the magnesium sample?
Report answer with correct units and sig figs.
a. 8.40 mL
b. 14.60 grams
250 for each question. For each one, sig figs and units are both with 75 points.
Hard level
What element is this? It has 31 neutrons.
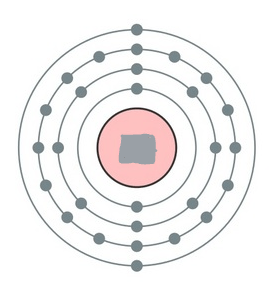
nickel, Ni