Chemistry is the ?
What is the the study of matter ?
What two numbers are used to identify a specific element in the periodic table?
What are the atomic number and mass number?
What is an ion?
What is an atom or molecule that has gained or lost one or more electrons, resulting in a net electrical charge?
If an object has a mass of 50 grams and a volume of 10 mL, what is its density
What is 5 grams per milliliter (g/mL)
Air is ?
What is a Homogeneous Mixture?
Isotopes of an element have the same number of protons but a different number of what other subatomic particle?
What are neutrons?
A Cation on is.
What is a positive metal ion.
If 1 inch equals 2.54 centimeters, how many centimeters are in 12 inches
What is 30.48 centimeters?
NaCl is a classification of what matter ?
What is a Pure substance/ Compound?
In which group of the periodic table can you find the noble gases?
What is Group 18 (VIII A or 8)?
What is a negatively charged atom.
Draw the orbital diagram for the element oxygen (O), which has 8 electrons
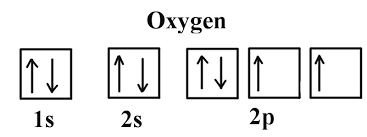
When a substance undergoes a chemical change, what type of change occurs in its chemical composition?
What is a chemical change?
what's the E-config 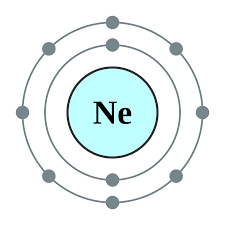
1s22s22p6
What is Sodium Chloride
An element has two naturally occurring isotopes: X-23 with a mass of 22.9898 amu and X-24 with a mass of 23.9850 amu. The abundance of X-23 is 30.0%, and the abundance of X-24 is 70.0%. Calculate the average atomic mass of this element.
Average Atomic Mass = (22.9898 amu × 0.30) + (23.9850 amu × 0.70)
Average Atomic Mass = (6.89694 amu) + (16.78950 amu)
Average Atomic Mass = 23.68644 amu
How many significant figures are in the number 0.00420?
What are 3 significant figures?
Write the electron configuration for the element nitrogen (N), which has an atomic number of 7.
What is 1s^2 2s^2 2p^3?
Binary name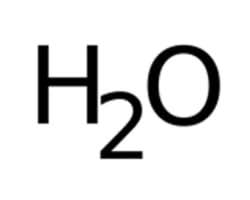
dihydrogen monoxide
An element has two naturally occurring isotopes, A-25 with a mass of 24.9858 amu and A-26 with a mass of 25.9826 amu. The abundance of A-25 is 20.0%, and the abundance of A-26 is 80.0%. Calculate the average atomic mass of this element.
Average Atomic Mass = (24.9858 amu × 0.20) + (25.9826 amu × 0.80)
Average Atomic Mass = (4.99716 amu) + (20.78608 amu)
Average Atomic Mass = 25.78324 amu