How many significant figures are in 350.2500?
7
Nathaly left her bicycle in the rain over night. The bicycle formed brown brittle spots. is this a chemical or physical change?
chemical change.
What phase of matter the substance at line CD?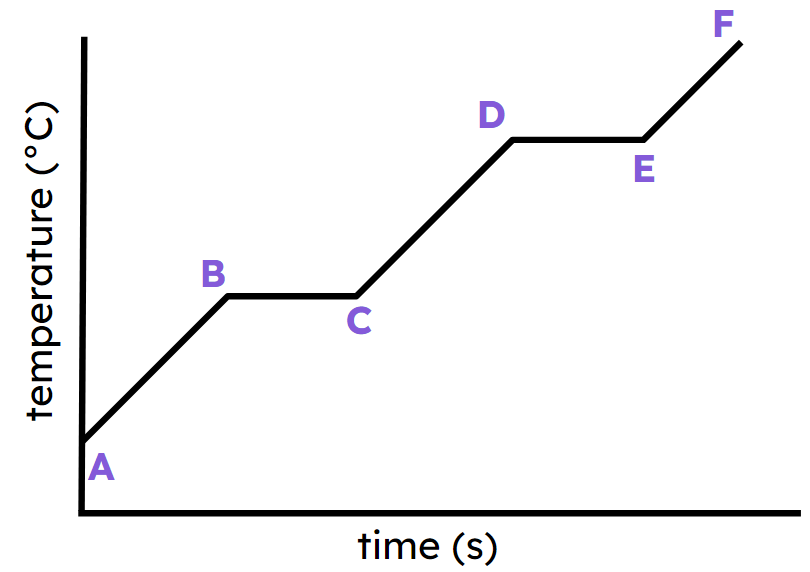
liquid
What is the charge, mass, and location of a proton
Positive charge, mass: 1 amu, located in Nucleus
What is the decay mode of Strontium-90?
beta decay
Convert 300 celsius to Kelvin
K= C + 273
K= 300+273 = 573K
Draw what the particles look like at line EF
How many protons and neutrons are in Au-198
79 Protons, 119 neutrons
Mass # = p + n
Which nuclear emission has no charge and no mass?
Gamma
42.9
answer in 3 sig figs
What type of matter is NaCl(aq)?
mixture
explain in terms of kinetic and potential energy what is happening in line BC.

Kinetic energy is constant,
Potential energy is increasing.
An atom in an excited state has an electron configuration of 2-6-3. Write the electron configuration of this atom in the ground state.
2-8-1
Compare the a fusion reaction to a fission reaction.
Fusion is when two smaller nuclei fuse to one bigger nucleus.
Fission is when a large nucleus is split into two or more smaller nuclei.
Emma who weighs 100 pounds, found a rock. What is the volume of the rock if it has a mass of 42g and a density of 7g/cm^3?
6cm^3
D=m/v, v=m/D
v=42/7
Explain the difference between a homogeneous and a heterogeneous mixture.
Homogeneous is when the substance looks the same. even distribution.
Heterogeneous is when there are different layers. Uneven distribution.
how many joules is required to melt 450g of ice at 0 degrees celsius?
150300 J
q=mHf
q=(450)(334)
What are the two conclusions of the gold foil experiment?
1. atoms are made mostly of empty space
2. atoms have a small, dense, positive center called the nucleus.
Write the nuclear equation for the decay of Rn-222.
There are 5.75 grams of sugar in Celeste's cookie. You determine the sugar content to be 4.0 grams in a lab. What is the percent error?
30%
Heat is added to a 200.-gram sample of H2O(s) to melt the sample at 0°C. Then the resulting H2O(ℓ) is heated to a final temperature of 65°C. Calculate the total amount of heat required to raise the temperature of the H2O(ℓ) from 0°C to its final temperature.
Q=mcΔ T
Q=(200)(4.18)(65)
Q=54340J
Explain in terms of electrons and energy levels how the light emitted from a flame is produced
when electrons in an excited state fall down to a lower energy level, light is produced.
If original there was 96.0g of Krypton-37, how much Krypton-42 remains after 6.15 seconds?
3 grams