Salt (NaCl) dissolved in water can be described as what type of matter?
(1) element
(2) compound
(3) homogeneous mixture
(4) heterogeneous mixture
(3) homogeneous mixture
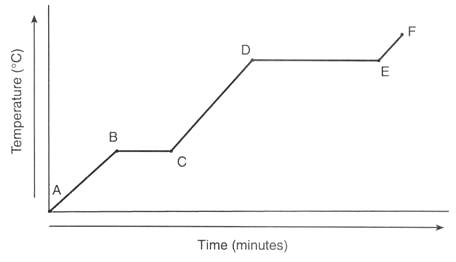 What is happening to the sample at line BC?
What is happening to the sample at line BC?
sample is melting
Which part of the atom has a positive charge?
(1) orbitals
(2) electrons
(3) valence shell
(4) nucleus
(4) nucleus
PROTONS ARE IN THE NUCLEUS.
Which statement below correctly describes what occurs in a nuclear reaction?
(1) Energy is lost as it is converted into mass
(2) Mass is lost as it is converted into energy
(3) Energy is gained as it is converted into energy
(4) Mass is gained as it is converted into energy.
(2) Mass is lost as it is converted into energy.
When a radioactive element decays, its matter breaks down and releases a HUGE amount of energy.
During all chemical reactions, we balance charge, mass and energy so they are all...
conserved!
Which type of matter would describe sand and water at room temperature?
(1) element
(2) compound
(3) homogeneous mixture
(4) heterogeneous mixture
(4) heterogeneous mixture

What is happening at line DE?
sample is vaporizing
Why are Ne-19 and Ne-20 isotopes of the same element, based on their subatomic particles?
Ne-19 and Ne-20 have the same number of protons in them- which means they isotopes.
Compared to a chemical reaction like combustion, a nuclear reaction has
(1) significantly less energy
(2) slightly less energy
(3) slightly more energy
(4) significantly more energy
(4) significantly more energy
Think about the nuclear power and the atomic bomb - nuclear energy is 100,000,000 times more efficient than burning coal for energy.
Based on its placement on the periodic table, why is NaCl an ionic compound?
NaCl is made of a metal and a nonmetal ion.
Which separation technique would work best to separate two liquids?
(1) filtration
(2) distillation
(3) chromatography
(4) evaporation
(2) distillation --> best for two liquids.
 Choose one point where only the kinetic energy of the substance is increasing.
Choose one point where only the kinetic energy of the substance is increasing.
AB, CD, EF
Based on the Periodic Table, which element has 92 protons?
U - Uranium
DAILY DOUBLE:
1. How many half lives have passed if a material goes from a fraction of 1 to 0.0625th of its material remaining?
2. If the half life of this material, according to table N is 5 years, how long will this material have been decaying for?
1 - 1/2 - 1/4 - 1/8 - 1/16
1 - 0.5 - 0.25 - 0.125 - 0.0625
Four half lives pass.
Based on the periodic table, what formula represents magnesium (ii) bromide?
(1) MgBr
(2) Mg2Br
(3) MgBr2
(4) Mg2Br3
(3) MgBr2
Think about the CROSS RULE. Mg2+ and Br-1 switch charges when determining the number of each element.
Table sugar can be separated from a mixture of table sugar and sand by using which method?
(1) filtration
(2) magnetism
(3) chromatography
(4) distillation at 100 degrees Celsius
(1) distillation
Distillation can separate a solid from a liquid by boiling the liquid. At 100 degrees C, the water boils.

Choose a line where only potential energy is increasing.
BC, DE
According to the Periodic table, how many valence electrons are in an atom with 12 protons?
An atom with 12 protons is Magnesium.
Magnesium has 2 valence electrons. Valence electrons are the LAST SHELL of electrons in an electron configuration.
In which type of nuclear reaction do two atomic nuclei form a single atom with a larger mass?
etc. H + H --> He
(1) Fusion
(2) Fission
(3) Alpha Decay
(4) Beta Decay
(1) Fusion
Two smaller elements combine to form a larger element.
What are the names of the two classifications of compounds we learned in class so far?
Hint: One TRANSFERS electrons; one SHARES electrons
Ionic - TRANSFERS electrons
Covalent - SHARES electrons

How do we know DE requires more energy than BC, according to the graph?
DE takes more time than BC
Based on the number of valence electrons, which ion is Sodium most likely to form? How many electrons will be transferred?
+1 charge; 1 electron will be lost
Sodium has 1 valence electron. Based on the octet rule, it wants to lose that one electron.
The ion formed will have a +1 charge.
Complete the following chemical reaction below:
23892U --> 14056Ba + _____ + 310n
9336Kr
There are THREE neutrons --> 238 = 140 + X + 4
--> 92 = 56 + X
Balance the following Chemical Equations
__K + __O2 --> __KO3
Recall - if you are balancing an even and odd number, balance by multiplying the opposite number on each side.
3 x 2 -- 2 x 3