what are the two types of ions?
Cation and Anion
Which way does ionization energy go AND what is the defintion.
up and right, amount of energy to remove an electron from an atom
What is Hubbles Law?
What are our 3 types of decays?
Beta, Alpha, gamma
If a star is moving away from earth what shift is that?
redshift
What charge would Phosphorous have as an ion?
-3
Which element has the largest atomic radius?
F, Si, Ge, Ba, Fr
Fr
What is Aufbau Principle
electrons first fill subshells of the lowest available energy
.png)
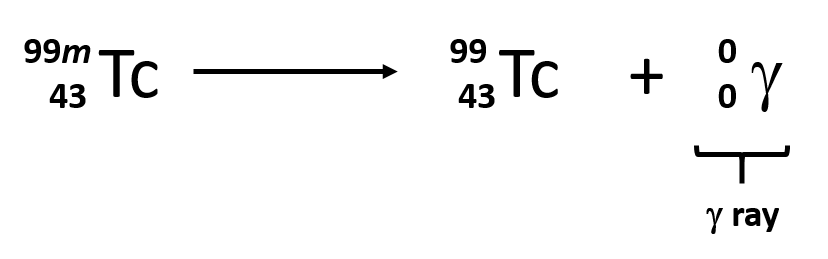
What type of decay is this?
Beta
Which shift has smaller wavelengths?
blue shift
What charge would Barium have as an ion?
+2
Which element has the smallest atomic mass?
N, F, Al, Sr
F
What is Pauli Exclusion Rule?
Orbatials can only hold a mximum of two electrons

what type of decay is this?
alpha
If a galaxy stays the same distance from Earth which shift is that?
Neither, no shift
Which of the following is a cation?
Na, O, Br, F
Na
Put the elements in order of smallest to largest ionization energy.
P, Si, Ba Fr, O
Fr, Ba, Si, P, O
What is Hunds Rule?
In a sublevel with more than one orbital put one electron in each orbital before moving on.
What is the doppler effect?
the increase (or decrease) in the frequency of sound, light, or other waves as the source and observer move towards (or away from) each other