The energy of blue light is _____ than the energy of green light because the higher the wavelength the ______ the frequency, decreasing the overall energy of that photon.
Greater, lower (lesser)
Write out in noble gas configuration the orbital notation for sulfur.
What is [Ne]3s23p4?
Describe the polarity of the following ion:
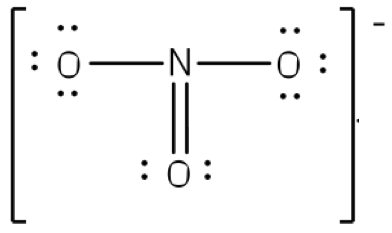
This is nonpolar due to resonance. Resonance means that there are equivalent Lewis Structures to describe this compound because in reality that double bond is equally distributed between the oxygens.
This occurs along the solid/gas line of a phase diagram.
What is sublimation/deposition?
What is the hybridization for a molecule with 3 electron groups.
sp2
What is the correct Lewis Structure for NCO-?
Explain the molecular and electron geometry of the second carbon in CH3CN.
What is linear, linear?
What are the general rules to filling orbital diagrams with electrons?
Fill lowest energy orbital first, spins in a single box must be in the opposite directions, half fill empty boxes before pairing electrons.
What does the sign of the enthalpy of reaction tell you about the overall reaction?
*Please indicate what each sign means
It tells you whether the reaction is endothermic (+) vs exothermic (-).
Draw the Lewis Structure with the formal charges for the ion I3-. What is the molecular geometry?
Molecular Geometry: Linear, formal charge -1 on central iodine. 
These four transition metals will either fill or half fill their d-orbitals before filling their s-orbital.
What are Cr, Mo, Cu, and Ag?
Define the terms of the ideal gas law. Must include units AS WELL AS the actual meaning of the variable.
P= pressure (atm)
V= volume (L)
n= molar amount (mol)
R= gas constant (L*atm/K*mol)
T= temperature (K)
This is the difference between electron geometry and molecular geometry.
What is the total number of electron groups rather the number of bonds vs lone pairs?
This is why we check whether a reaction equation is balanced before we begin stoichiometric conversions.
What is the correct molar ratios between species?
or
How do we get the correct molar ratios to perform stoichiometric conversions?
Is bond breaking endothermic or exothermic?
Endothermic
*This is not the same as energy released after bonds break, this is the energy you put in to break the actual bonds
Why do atoms hybridize?
To lower energy
Balance the equation:
C2H4(g)+O2(g)-->CO2(g)+H2O(l)
C2H4(g)+3O2(g)-->2CO2(g)+2H2O(l)
Put in order of increasing bond angles: NH3, H2O, CH4
H2O<NH3<CH4
When observing an object emitting light, what subatomic processes is responsible for what you eyes see?
Electrons moving from high energy states to lower energy states.
This is the Pauli Exclusion Principle.
Why can't 2 electrons have the same spin in the same orbital?
If the atomic mass of silicon is 28.0855 amu, which of its isotopes do you expect it to be more abundant?
Si-28, Si-29, or Si-30?
(Reason through this, don't calculate)
Does not need to be in the form of a question!!!
Si-28 because the atomic mass is closest to 28 amu.
As we know, the ideal gas law makes several assumptions about gases to make predictions about their behavior easier. Knowing these assumptions, this is why water isn't an ideal gas.
DOUBLE JEOPARDY!!!!
How does polarity affect the behavior of gases?
Water is a polar molecule that can form hydrogen bonds with itself. This means that there is actually interaction between the molecules.
*You should be able to connect concepts from one portion of the course to another: I used IMF to explain why water would not behave ideally.
Draw a sigma bond vs a pi bond. Use this drawing to explain why one is stronger than the other.

Because the sigma bond is formed from direct orbital overlap, it is stronger than a pi bond. A pi bond is formed by aligning the orbitals parallel with each other and having the electrons form a bridge between them. Since the orbitals aren't in direct contact, their association isn't as strong.
When energy is released, we describe the reaction as this.
What is exothermic?
Which bond has more overlap, pi or sigma?
The sigma bond.
When the energy needed to form a bond is greater than the energy needed to break a bond in a chemical reaction, would it be exothermic or endothermic?
Exothermic
Describe an electron in the 3s orbital using quantum numbers.
Example: n=3, l=0, ml=0, ms=1/2
What are the two points (the dots) on the diagram? What is occurring at these points?

The triple and the supercritical points. At the triple point, all three phases happen at once. At the supercritical point, we can no longer tell the difference between a liquid and a gas.
What are the formal charges on the atoms in HF?
H: 0, F: 0
What is the significance of ψ2 at a particular point in an atomic orbital?
a) ψ2 determines how many electrons are present at that point
b)ψ2 determines the probability of finding an electron at that point
c)ψ2 determines the average velocity of the electron at that point
d)ψ2 determines how many electrons in total are present in the atom
e)ψ2 has no significance at any particular point in an atomic orbital
What is B?
This is the equation for root mean squared:
v_(rms)=sqrt((RT)/(M))
It is the description of the speed of a gas (vrms) according to temperature (T) and molar mass (M).
Which R do you used for this equation, what units for mass do you use, and what happens to speed if you increase molar mass?
DOUBLE JEOPARDY!!!
You use 8.314 J/mol*K for R.
You use kg/mol for molar mass units.
If you increase molar mass, speed decreases.
Which of the following statements is not true? (Double Jeopardy)
a) Molecular orbitals are formed by the linear combination of atomic orbitals.
b) An antibonding molecular orbital concentrates electron density between atoms.
c) A molecule formed from atoms that have a total of n atomic orbitals will have n molecular orbitals.
d) An antibonding molecular orbital has one or more nodes.
e) The antibonding molecular orbital formed from a pair of atomic orbitals lies at higher energy than the bonding molecular orbital.
DOES NOT NEED TO BE IN THE FORM OF A QUESTION!!!
Answer: B
This is the exact opposite of what an antibonding orbital does. The concentration of electron density indicates the formation of a bond.
What are the rules to determining the enthalpy of a final reaction when deriving from intermediate reactions?
1. If a reaction is multiplied by a factor, the enthalpy that reaction must be multiplied by the same factor.
2. If a chemical reaction must be reversed, so too must the sign of that reaction's enthalpy.
3. If an overall chemical reaction can be expressed as a series of simpler chemical reactions, then the enthalpy of the overall chemical reaction is the sum of the enthalpies of the simpler reactions.
Order these from the least amount of energy to the greatest: visible light, x-ray, microwave, gamma, infrared, radio, UV
Radio, microwave, infrared, visible light, ultraviolet, x-ray, gamma
What type of process is bond formation?
Exothermic
What are the three parts of the kinetic molecular theory?
Size of the particle is negligibly small.
Kinetic energy is proportional to temperatureCollisions are elastic
What is the difference between nuclear fission and nuclear fusion?
Fusion: light elements fuse to for heavier elements.
Fission: heavy elements split to form lighter elements.
When the energy of the bonds broken is greater than the energy of the bonds formed, this describes the reaction.
What is endothermic?
What is the molecular and electron geometry of NH3? What is the hybridization?
Tetrahedral, trigonal pyramidal, sp3.
What are the two formulas for wavelength?
lamda=c/v
lamda=(hc)/E
Place the following in increasing atomic radius: As, O, Br
As>Br>O
This principle explains why we can't determine the position and velocity of a particle.
What is the Heisenberg Uncertainty Principle?
Put in order from the least to the most electronegative: Ca, N, K, Cl
K<Ca<N<Cl