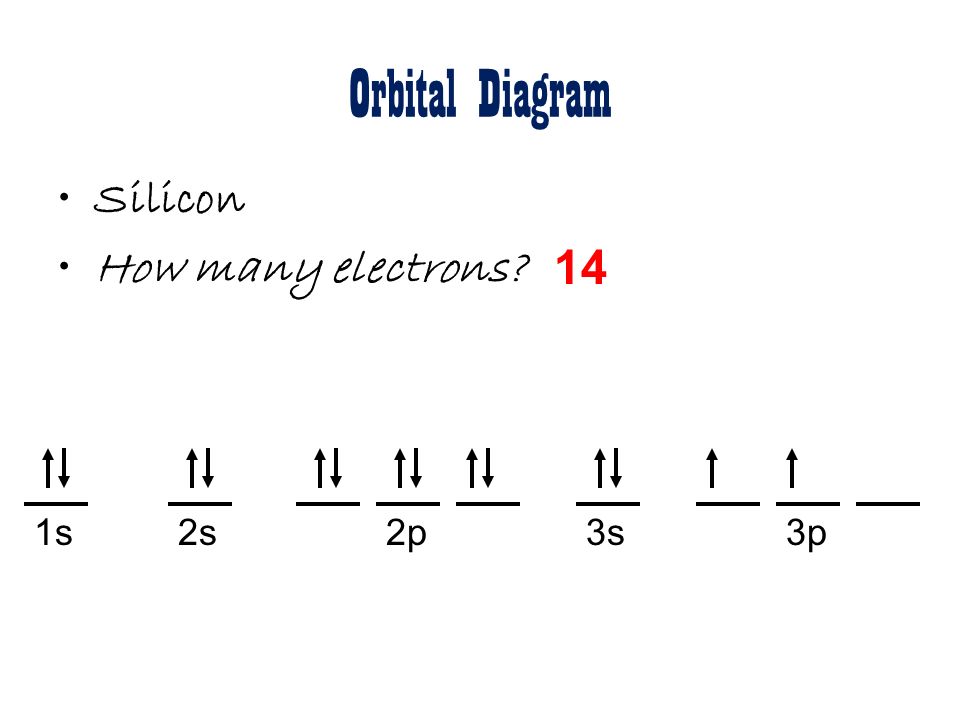
Draw out the orbital notation electron configuration for a neutral silicon atom.
What is the main reason that atoms form ions?
a. to bond with other elements
b. to create a negative charge
c. to develop a positive charge
d. to fill their outer electron shells
d. to fill their outer electron shells
Which of the following correctly shows the bond polarity?
a. 𝛅+P - N𝛅-
b. 𝛅+I - I𝛅-
c. 𝛅+F - Cl𝛅-
d. 𝛅+O - S𝛅-
a. 𝛅+P - N𝛅-
electron configuration in atoms
Iran

The ground state electron configuration for an element contains an unpaired 3s electron. Which of the following is the identity of the element?
a. Li
b. Na
c. Mg
d. K
b. Na
The compound KBr is commonly used in veterinary medicine to treat epilepsy in dogs. On the basis of periodic properties, which of the following compounds can most likely also be used to treat canine epilepsy?
a. CBr4
b. NaBr
c. C3H8O
d. K
b. NaBr
Which of the following correctly lists the substances HCN, N2, and HF in order of increasing IMFs?
a. HCN < N2 < HF
b. N2 < HCN < HF
c. HF < N2 < HCN
d. N2 < HF < HCN
b. N2 < HCN < HF
What was the purpose of this lab?
Observe differences in MP and conductivity in ionic and covalent compounds
Peru

In which of the following ionic compounds is the force of attraction between the cation and anion the strongest?
a. RbI
b. CsI
c. MgO
d. SrS
c. MgO
The Lewis structure of vinyl fluoride is shown on the right. Which of the following identifies the strongest intermolecular force present among molecules of vinyl fluoride?
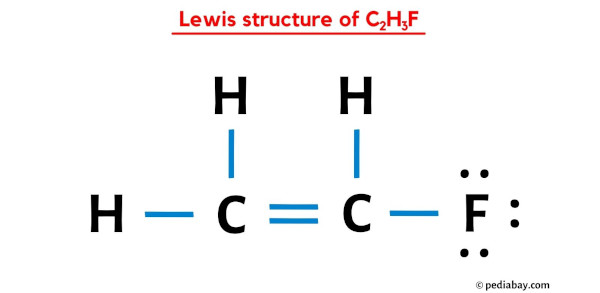
a. LDF
b. Dipole-Dipole
c. Hydrogen Bonding
d. Ionic Bonding
b. Dipole-Dipole
In class, you used these modeling kits. The models were used to help you better understand what theory?
Valence Shell Electron Pair Repulsion (VSEPR) Theory
Panama

Which rule states that electrons in the same orbital cannot have the same spin?
Pauli exclusion principle
Every electron has an associated spin, similar to the way a top spins on its point. Like a top, an electron is able to spin in only one of two directions.
The electron configurations of four different elements are shown below.
Element 1: 1s22s22p4
Element 2: 1s22s22p5
Element 3: [Ne]3s23p1
Element 4: [Kr]5s1
Based on their electron configurations, which of the following two elements are most likely to form an ionic compound with the formula X2Y?
Element 1 and Element 4
Draw the Lewis Dot Structure and use the VSEPR theory to predict the shape and bond angle of the nitrate ion.

trigonal planar, 120 degrees
We watched videos showing various alkali metals and their reactivities in water. Explain why reactivity of alkali metals increases as one moves from top to bottom of the periodic table. You must describe two periodic trends to support your explanation.
Atomic radii increases going down the group because more electrons occupy more orbitals, which causes ionization energy to decrease going down the group because the valence electrons are farther from the nucleus and are therefore more easily removed. This causes reactivity to increase going down the group
Finland
The ground state electron configuration for an element is [Kr]4d105s2. You may also see this electron configuration written as [Kr]5s24d10. Which of the following is the identity of the element?
a. Zn
b. Kr
c. Sr
d. Cd
d. Cd
Sodium, a reactive metal, and chlorine, a poisonous gas, can be combined to form table salt. Using chemistry terminology, discuss the formation of table salt and explain why this new substance is mostly non-reactive. Be sure to include the chemical names and formulas of everything involved in the reaction - this includes ions and neutral substances. (4 points - all or nothing)
Sodium, Na, loses an electron to form Na+. (1 point)
Chlorine, Cl, gains an electron to form Cl-. (1 point)
Na+ and Cl- are attracted to each other (1 point)
and make the neutral compound, NaCl, called sodium chloride (1 point)

Explain why some VSEPR shapes have smaller bond angles, even though they have the same number of electron densities.
lone pairs of electrons occupy more space around the atom
so they will push the neighbors farther away
In the activity - The Name’s Bond, Chemical Bond: Agent Forever Chemical - which chemical did we study? Why is this chemical causing so many problems?
PFAS - made up of many CF bonds, which are strong and difficult to break, causing PFAS to persist in the environment for a long time
Madagascar