Fill in the blank: Substances that decay have a measurable __________ that can help us determine how old they are.
Half life!
What is the name for the center of the atom?
The nucleus.
Fill in the blank: The _________ of a tool lets us know how precise it can be and helps us pick where to round to in addition problems
Resolution!
Fill in the blank: The strength of _________ between particles determines the temperatures at which it will change phase.
Intermolecular forces (or cohesion)!
The reason that water doesn't act like hydrogen or oxygen, even though it is made of hydrogen and oxygen.
Chemical bonds!
How do engineers keep a fission nuclear reactor from melting down?
They use substances that absorb neutrons to slow down the chain reaction.
Fill in the blanks: The __________ force affects anything that has _______.
Electromagnetic force - charge
Why do scientists care whether a measurement is written "2" grams or "2.0" grams?
Significant digits help scientists understand the strength of the tools used to make the measurement.
Which would contain more atoms, a mole of water (H20) or a mole of hydrogen (H2)?
Water: one water molecule has 3 atoms while one hydrogen molecule only has 2 atoms
Fill in the blanks: a chemist would call water a _______ and the particles that make it up ________
What daughter nucleus would be left over after Uranium-235 goes through alpha decay?
Thorium-231!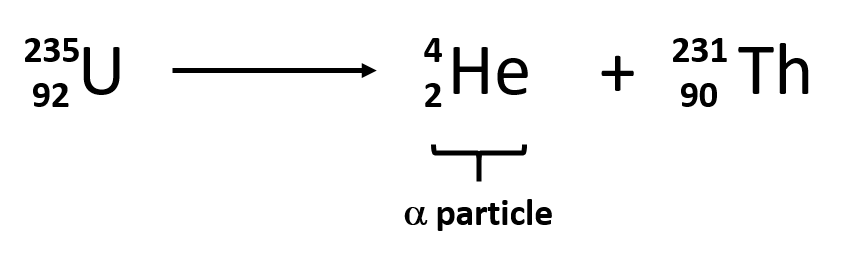
What is the most common isotope of the element carbon?
Carbon-12
How many square centimeters (cm2) could fit in a square meter (m2)?
10,000 cm2 = 1 m2
Measure out a mole of any substance in the room and bring it to Mr. R to earn these points.
Use the periodic table and a scale.
Why is KMT a theory, not a law?
It explains why things occur.
Polonium-214 has a relatively short half-life of 164 seconds. How many seconds would it take for 8.0 g of this isotope to decay to 0.1 g?
What is the charge of an atom of Carbon with 5 electrons in its orbitals?
6 protons - 5 electrons = +1 charge
What is the correctly rounded mass of 75.0 mL of a substance with a density of 1.834 g/mL?
138 grams!
The mass of 1 million atoms of gold.
What is 3.2*10^-16 grams?
Measure out the smallest amount of particles you can using a scale, then prove how many you have.
What substance did you choose?
Write a CER style response: Explain which type of decay (alpha, beta minus, or beta plus) is most likely to occur in one of the four regions shown below. 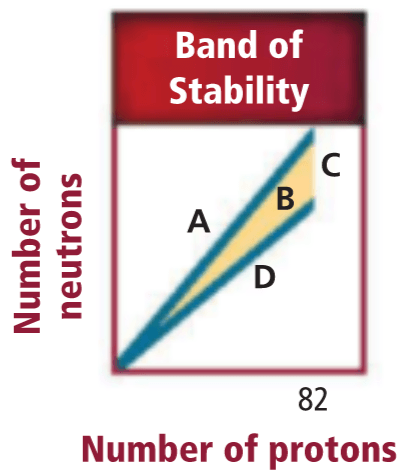
Make a claim
Back your claim up by reading the x and y axis of the graphs.
Explain why that type of decay would fix the imbalance of protons and neutrons.
207 mass - 82 protons = 125 neutrons
256 googs = 1 plotz
1 wraslm = 12.3 plotz
3.4 tpocks = 1 mulm
How fast is 4.27 googs/mulm in wraslms/tpock?
0.000399 wraslms/tpock
10 grams * 1 mole / 18 grams * 6.022*1023 particles / 1 mole * 3 atoms / 1 particle
Write a CER style response: which lab, activity, or assignment will help you the most on this upcoming test?
Choose an assignment to include as your claim. Write some evidence that makes you think that material will be on the test. Explain in reasoning why that activity was useful for learning that material.