What elements are in a hydrocarbon?
Hydrogen and carbon
Electrons
Using Table G, a solution at 50 oC that contains 70 g of NaNO3 is considered:
a) unsaturated
b) saturated
c) supersaturated
A) unsaturated because the point for 50 C and 70 g is under the line for NaNO3
How much a 400 g sample of Co-60 will remain after 15.813 years?
15.813/5.271 = 3 half lives
400--> 200 --> 100 --> 50 g
Draw the Lewis Dot Diagram for boron
B with 3 dots on different sides
What is the empirical formula of C4H10?
C2H5
What is the pH range of a solution that turns blue in bromthymol blue?
7.6-14
Calculate the molarity of a solution that contains 5 moles of solute in 250 mL of solution.
5/0.25 = 20 M
Calculate the amount of heat needed to vaporize 34 g of water.
q = mHv
q = 34 x 2260 = 76,840 J
When a reaction's forward rate is equal to its reverse rate, this is called
equilibrium
Label each of the following compounds as alkanes, alkenes, or alkynes:
C5H8 C2H4
C10H22 CH4
C5H8 alkyne C2H4 alkene
C10H22 alkane CH4 alkane
What is the chemical formula for the substance that forms when a hydrogen ion attaches to a water molecule?
H3O+
Fish breathe by taking in dissolved oxygen gas from the water. Explain whether the fish would have more available oxygen in warm water or cold water and why.
There would be more dissolved oxygen in cold water because gases dissolve better in cold water.
Determine the number of grams of K2O in 12 moles if the gram-formula mass is 94.2 g/mol.
12 = x/94.2
x = 12 x 94.2
x = 1130.4 g
State whether the compound below has ionic or covalent bonds AND whether the electrons are shared or transferred.
K2O
Ionic (M+ NM) and transfer
Draw the structure of hexane.
CH3CH2CH2CH2CH2CH3
How does the amount of H+ and OH- compare in:
acids?
bases?
neutral substances?
acids: more H+
bases: more OH-
neutral: equal amounts of both
1. You are making lemonade by adding lemonade powder to water until it is very sweet but you do not see any powder at the bottom of the solution.
2. You then decide to add a little more lemonade powder which falls to the bottom of the solution and does not dissolve no matter how much you stir.
Explain the level of saturation of the solution after step 1 AND after step 2.
After Step 2 - the solution is now supersaturated because it contains more solute than can be dissolved.
3000T = 1459500
T = 486.5 K
What is the percent composition of oxygen in KMnO4 if the gram-formula mass of the compound is 158 g/mol?
(4 x 16) / 158 = 0.405 x 100 = 40.5%
Name this molecule: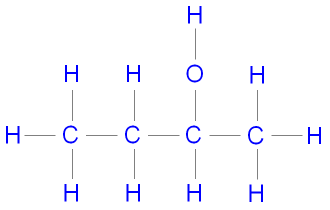
2-butanol
What are the oxidation states for each of the elements below?
HNO3
H = +1
N = +5
O = -2
Draw a molecule of KBr that has been dissolved in water. Be sure to show charges and draw at least 4 water molecules with partial charges.
K+ and Br-
The waters should align so that the O is closest to the K and the H's are closer to the Br.
First, balance the equation below. Then calculate the number of moles of Sr(OH)2 that is needed to make 8 moles of H2O.
__ Sr(OH)2 + __ HCl --> __ SrCl2 + ___ H2O
_1_ Sr(OH)2 + _2_ HCl --> _1_ SrCl2 + _2__ H2O
1 mol Sr(OH)2 = 2 mol H2O
x 8 mol H2O
8 = 2x
x = 4 moles Sr(OH)2
Draw BOTH the structures of 2-pentanol and 1-pentanol. Are these isomers, different, or the same molecules and why?
Isomers - same formula but different arrangement (OH) on different carbons.