What is the smallest unit of matter that cannot be broken down further by chemical means?
What is an atom
This separation technique is used to separate the components of a mixture based on the differences in their boiling points
What is distillation?
Which phrase describes the protons and neutrons in atoms of two different isotopes of the same element?
The same number of protons and the same number of neutrons
The same number of protons and a different number of neutrons
A different number of protons and the same number of neutrons
A different number of protons and a different number of neutrons
The same number of protons and a different number of neutrons
6.02 x 1023
What is Avagadro's number?
Matter is defined as anything that occupies space and has?
Which subatomic particle carries a positive charge?
What is a proton
Which particle diagram represents one substance only?
Particle A
How many chlorine atoms are in FeCl3?
2
6
3
4
3
What is the atomic mass of H2SO4?
98 grams
When sugar is dissolved in water, the resting solution is classified as a:
Homogenous mixture
Which two particles have a mass approximately equal to one atomic mass unit?
What are protons and neutrons?
According to Reference Table H, what is the temperature of ethanoic acid at 80 kPa?
What is 111℃?
Which scientist discovered electrons based on a “plum-pudding model”?
Who is Thompson?
The empirical formula for ethylene is CH2. Find the molecular formula if the molecular mass is 28.1 g/mol?
C2H4
Which is a formula of a binary compound?
1) BiPO4
2) BaSO4
3) Mg(ClO)2
4) MgCl2
MgCl2
How many molecules of water are indicated by the formula?
2 molecules of water
Which two particle diagrams represent two different phases of the same compound, only?
B and D
What two gases make up this unknown?
Gas A & D
How would you balance this chemical equation?
2 Al + 3CuCl2 ---> 3Cu + 2AlCl3
The particle diagrams below represent elements at STP. Which particle diagram best represents a substance in the solid state? 
A
Working in the laboratory, a student finds the density of a piece of pure aluminum to be 2.85 g/cm3 . The accepted value for the density for the aluminum is 2.699 g/cm3 . Determine the percent error
What is 5.59%
Which sample of matter is classified as a mixture?
NaCl (s)
CH3OH (l)
SO2 (g)
4. KNO3 (aq)
4. KNO3 (aq)
What does the lewis-electron dot diagram for an atom of Aluminum look like?

What is the percent composition of calcium in CaCl2?
Ca = 40.1g x 1 = 40.1 g
Cl = 35.5 g x 2 = 71.0 g
GFM = 111.1 g/mol
% Composition = mass of part / mass of whole x 100 % comp = 40.1 g / 111.1 g/mol = 36.1%
Ans: 36.1%. This means that Ca is 36.1% of the entire compound of CaCl2 which is 111.1 g/mol
What general type of reaction is illustrated in the diagram?
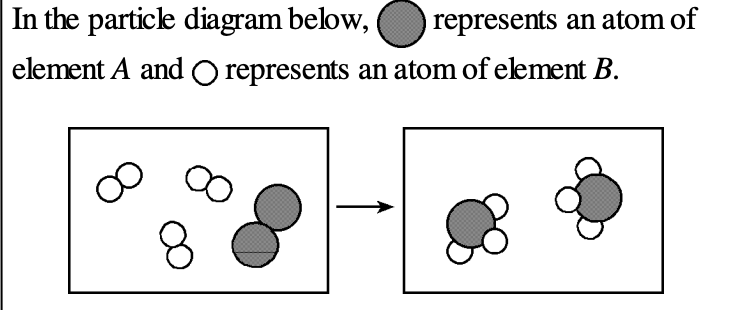
a) synthesis
b) decomposition
c) single replacement
d) double replacemnet
Synthesis