These two pieces make up the units for the rate constant
What are molarity(concentration) and time?
The probability of finding a particle at a node in a wave-function.
What is zero?
The probability of finding the particle within the first two peaks for the n=3 state.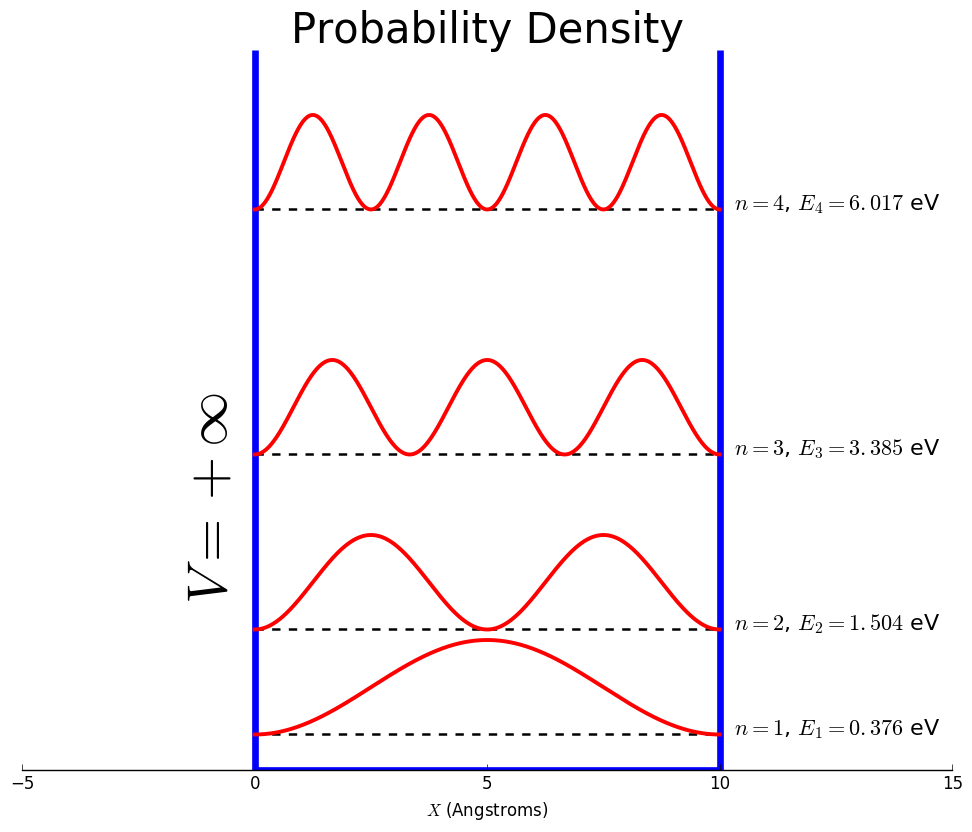
What is 2/3?
The major requirement for a molecule to have a rotational spectrum.
What is a permanent dipole?
v = (1/2π)√(k/μ)
What is the formula for the harmonic frequency?
The order of the reaction based on this diagram
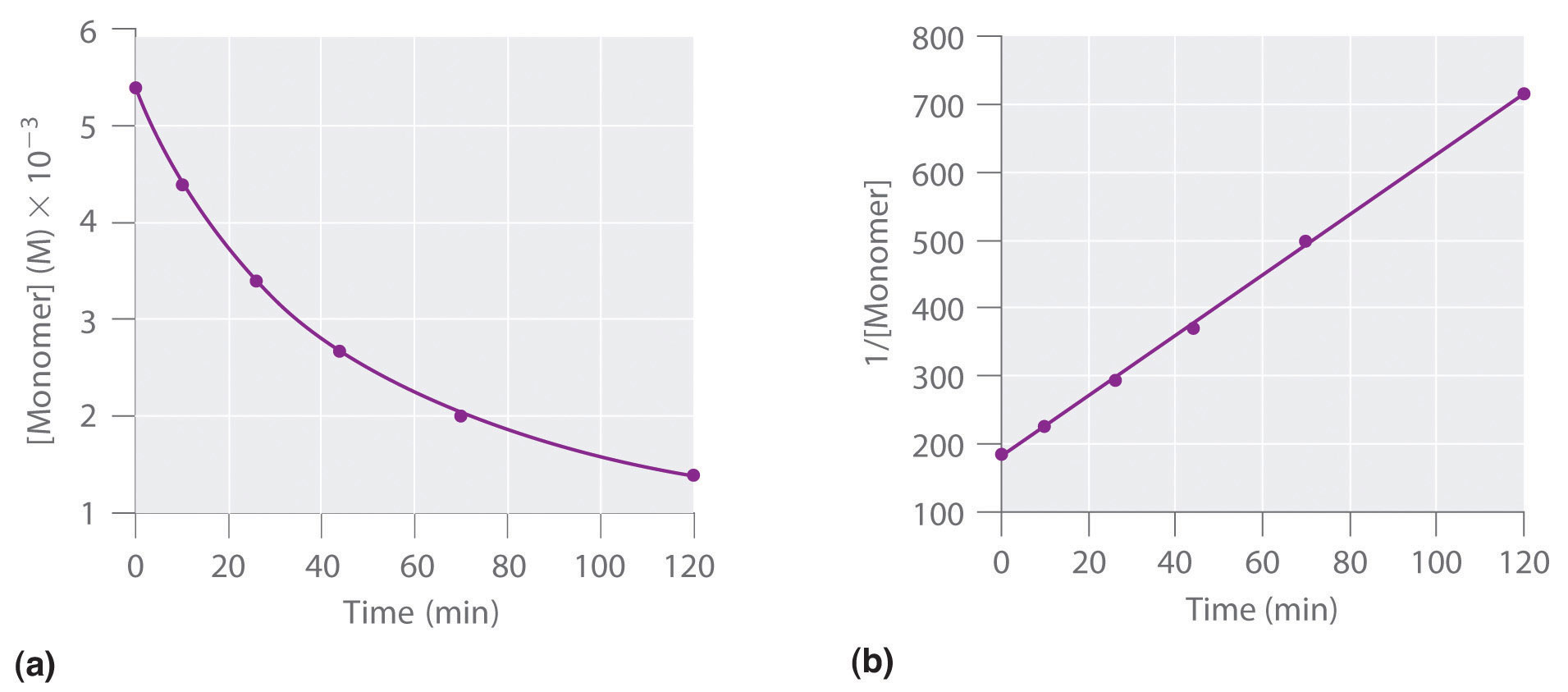
What is second order
The conditions for the formation of molecular orbitals.
What is symmetry and similar energy?
The model for the shown wavefunctions.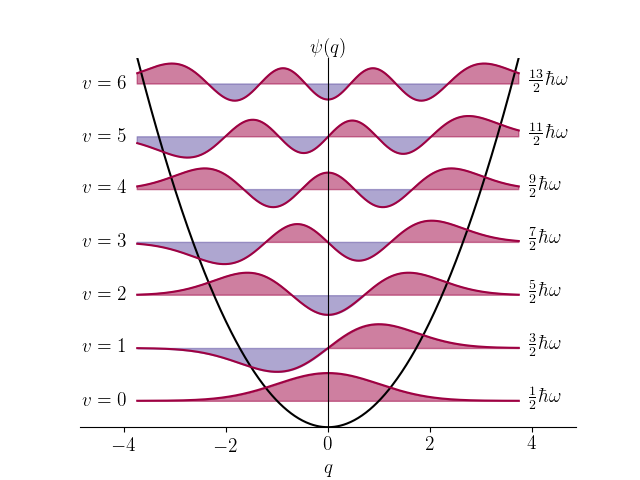
What is the harmonic oscillator model?
The type of light associated with molecular vibrations.
What is IR light?
ΔE= 2h(J+1)Be
What is the energy for a rotational transition?
These species are both made and consumed during a reaction mechanism
What is an intermediate?
The ionization energy for a Be3+ atom.
What is -217.6 eV?
The De Broglie wavelength for an electron moving at half the speed of light.
What is 4.85x10-12 m?
This value dictates chemical shift in NMR
What is electron shielding?
nλ = 2d sin(θ)
What is Bragg's equation?
N2 + 3F2 -> 2NF3
F2 decreases at ______ the rate which NF3 increases
What is 3/2
The three conditions for a well behaved wave-function.
What is single valued, continuous, and quadratically integrable?
This part of the Hamiltonian prevents us from mathematically solving the Schrodinger equation for many electron atoms.
What is the electron-electron repulsion piece?
The seven types of crystal lattices
What is Triclinic, Monoclinic, Orthorhombic, Rhombohedral, Tetragonal
, Hexagonal, Cubic
ve-2JBe
What is the frequency of the roto-vibrational transition?
The graph below shows the time-dependent change in the concentration of NO. If NO decomposes at 520 °C based on the following equation,
If NO decomposes at 520 °C based on the following equation,
2 NO(g) → N2(g) + O2(g)what is the average rate of consumption of NO over the time interval 0—40 s?
What is 0.005 M/s?
The energy of the 2nd vibrational excited state for a molecule with a fundamental frequency of 5.00x1013 Hz.
What is 8.28x10-20 J.
The wave function for the n=3 state for a particle in a box.
What is ψ3= √(2/l)sin(3πx/l)
The selection rules for the vibrations of polyatomic atoms.
What is the change in vj = ±1 and the jth normal mode vibration must change the molecular dipole moment
=(n2h2)/(8ml2)
What is the energy for particle in a box?