A game of tug-of-war, in which two teams pull on opposite ends of a rope. The center of the rope will move when one team....
pulls with more force.
Heat transfers from...
Hot to cold
True or false: Metalloids can be found on the staircase/zig-zag line of the periodic table.
True.
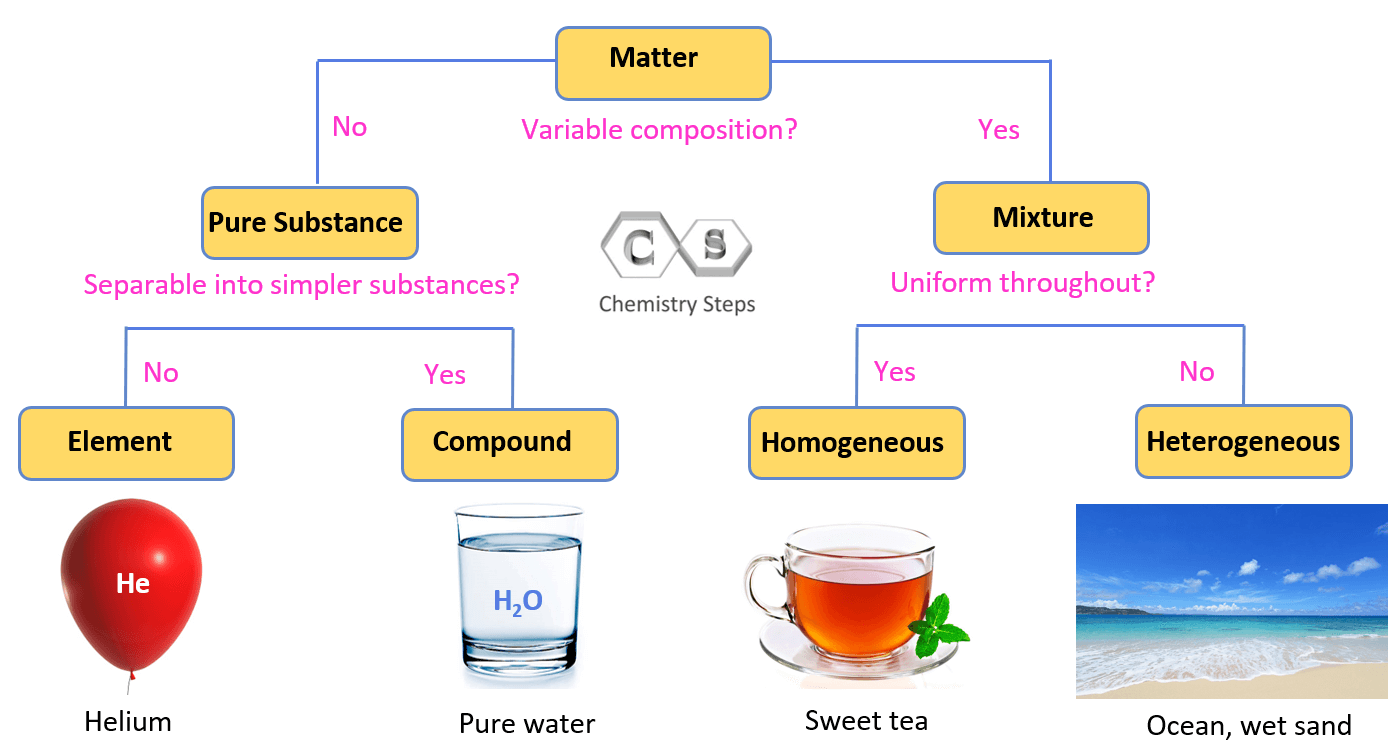
True or False. A pure substance is a homogeneous mixture.
False, it would refer to an element or a compound.
True or false: 2 Oxygen atoms together is a compound.![]()
False. It is an element because it is only one kind of atom.
A box rests motionless on the ground. One student is pushing the box to the RIGHT with a force of 2 Newtons. The other student is pushing to the LEFT with a force of 5 Newtons. In which direction and with what type of speed will the box move?
Left, 3N
True or False: Conduction is the flow of heat inside an object.
True
Where would a student find an element is malleable and has a luster in the periodic table? *Think if it would be metal, metalloid, or nonmetal.

Metal, left side and middle of periodic table.
A cup of lemonade is an example of which type of mixture?
Homogeneous
Elements can be found on the
Periodic Table
If an object travels 42 meters in 6 seconds, what is its average speed? (Speed=Distance/Time)

7m/s
 Which energy transformation occurs in this image?
Which energy transformation occurs in this image?
Radiant to Chemical
If an element is NOT malleable and a poor conductor, is it a metal, nonmetal, or metalloid?
Nonmetal
A bowl of chicken noodle soup is an example of which type of mixture?
Heterogeneous
The smallest particle of any particular type of matter is known by which of the following terms?
Atom
A soccer ball takes 30 s to roll 15 m. Calculate the average speed of the ball using distance and time measurements. (Speed=Distance/Time)
0.5s/m
True or False: Convection is the flow of heat between objects that are not in contact with each other.
False. It is Radiation.
If an element is a semi-conductor and has a luster to it, is it a metal, nonmetal, or metalloid? Where are they located?
 metalloids, by the zig zag/staircase
metalloids, by the zig zag/staircase
Compare a homogenous and heterogenous mixtures.
A homogenous mixture become one new uniform substance. A heterogeneous mixture is not a uniform substance.
Carbon is considered an element, while carbon dioxide is considered a compound. This is because carbon dioxide is –
made of two different elements.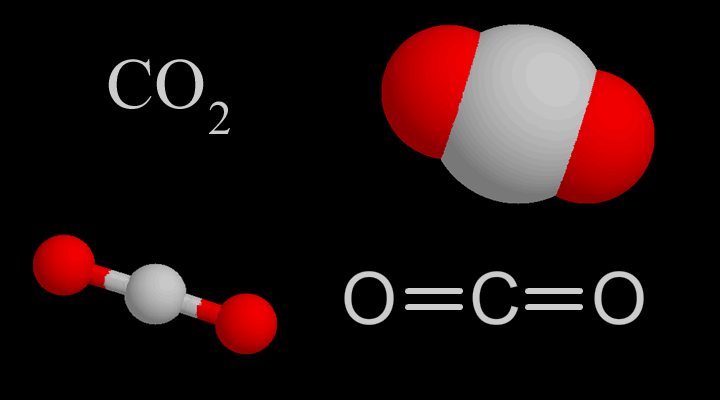
Newton's 1st Law states that "an object at rest will remain at rest until another force acts on it." Explain a situation proving Newton's 1st Law.
Any answer describing Newton's 1st Law/a balanced force becoming an unbalanced force.
At which point is potential energy converting to kinetic energy? 
Student answers may vary
Student should name one for each category.
Give an example of both a homogeneous and a heterogeneous mixture. Be sure to clarify which one is which. (It cannot be one mentioned in any of the questions above)
Must be a clear example of both mixtures.
How are elements and compounds different?
A compound is represented by a chemical formula, and elements are represented by a chemical symbol.
