True or False: A taxa 1 benthic is tolerant to pollution
FALSE.
Taxa 1 means pollution intolerant
What are the two types of mixtures and give an example of each?
Heterogenous (beef stew) and homogeneous (cough syrup)
Which state of matter has no definite shape or volume and the highest kinetic energy?
Gas
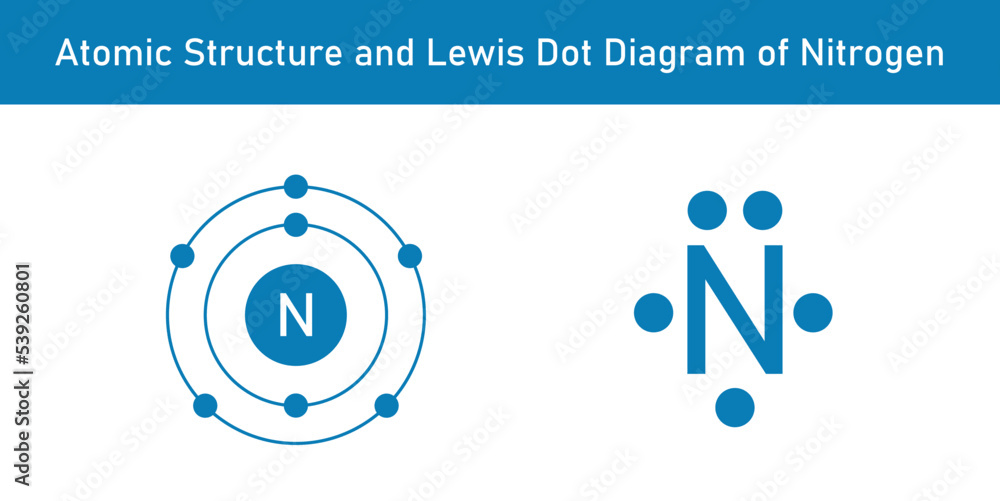
Draw the Bohr Diagram and Lewis Dot Structure for Nitrogen.
Name the following ionic compound:
Na2CrO4
Sodium chromate
Define non-point pollution and give an example
Non-point pollution is pollution that cannot be traced to a single source. An example is road salt or runoff
Which type of change, is easily reversible, and includes things like crushing, cutting, and melting?
Physical
What type of energy is increasing on the sloped parts of a heating curve and is a measure of the temperature?
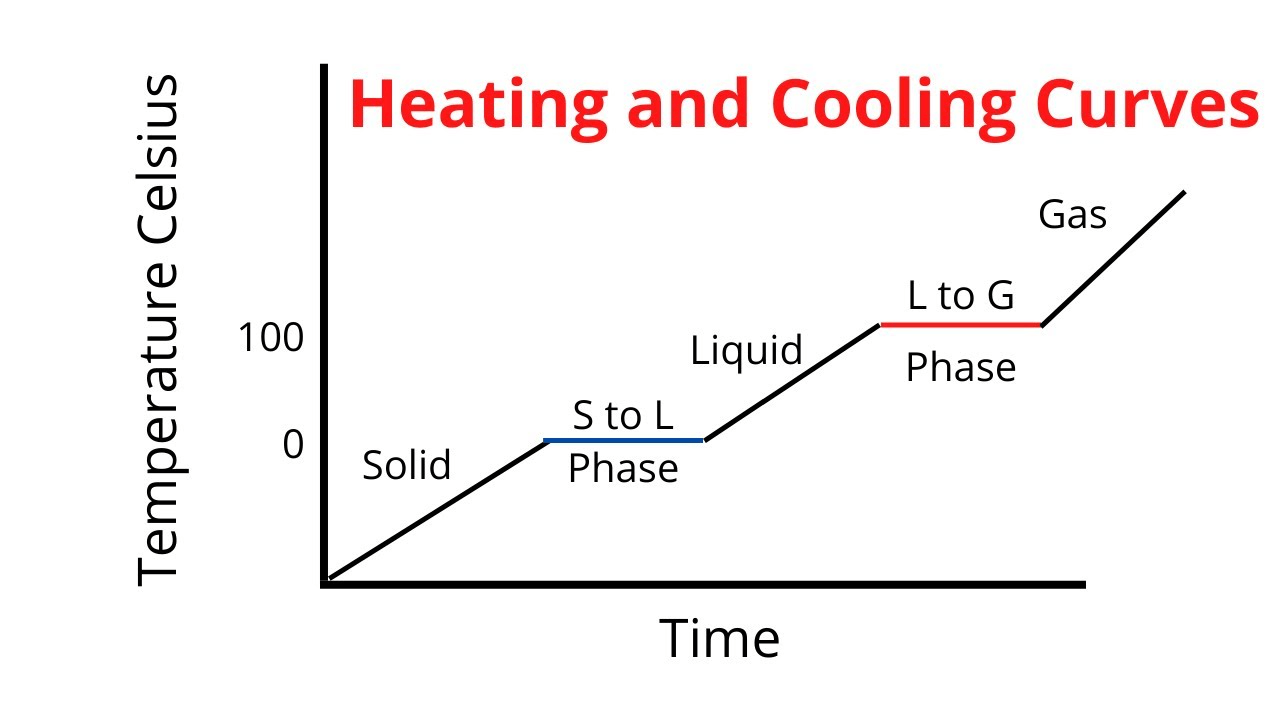
Kinetic Energy
The atomic number for Carbon is 6 and the atomic mass is 12. How many of the following does Carbon have:
Protons
Neutrons
Electrons
Name the following compound:
PbCO3
Lead (II) carbonate
What is defined as all of the species in one place at one time?
A Community
Lighting a firecracker is an example of what type of change?
Chemical Change
At what temperature does water freeze and melt?
0 degrees
What is the oxidation number of Oxygen and how many valence electrons does it have
Oxidation number: -2
Valence e-: 6
Write the following compound:
Ammonium phosphite
(NH4)3PO3
A cockroach has many offspring, no parental care, and a short life span. What type of strategist is a cockroach?
An r-strategist
What is the purest form of matter?
An element
20g of liquid water is heated from 20-40 degrees. How many calories are lost in this 1-step process?
20g x 20 C x 1 = 400 calories
Why are atoms considered electrically neutral?
They have the same number of protons and electrons
Write the following compound:
Copper (II) chloride
CuCl2
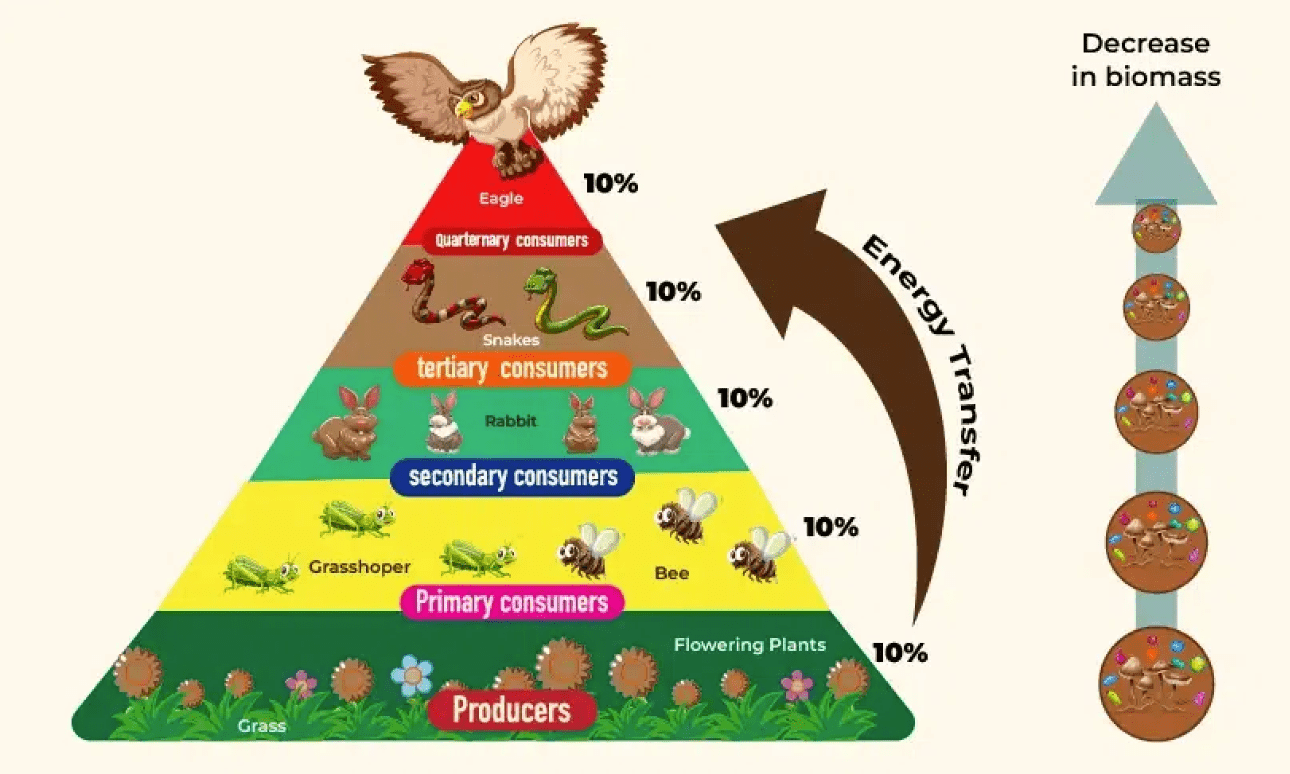
How much energy (what percentage) is lost between each trophic level on an energy pyramid
90%
Water (H2O) is considered what type of matter?
Compound
A 10 g solid chuck of ice is heated from -50 to 0 degrees. Once it hits 0 degrees, it melts into liquid water. How many calories are lost in this two step process?
10g x 80 = 800 calories
TOTAL = 1050 calories
What is the name of an atom that has gained an electron?
An anion
Ammonium hydroxide + Beryllium oxide -> Ammonium oxide + Beryllium hydroxide
2 NH4OH + BeO -> (NH4)2O + Be(OH)2