Type of mixture in which the individual substances are not evenly mixed _____________
A. element
B. homogeneous mixture
C. compound
D. heterogeneous mixture
D. Heterogeneous Mixture
Which statement refers to a physical property of a substance?
A. Hydrogen combines with oxygen to form water.
B. The density of copper is 8.93 grams per cubic centimeter.
C. Baking soda reacts with vinegar to produce a gas.
D. Acids are corrosive.
B. The density of copper is 8.93 grams per cubic centimeter.
The change of matter from a liquid to a gas.
A. vaporization
B. condensation
C. sublimation
D. deposition
A. vaporization
A characteristic of matter that allows it to change to something new is a____________.
A. chemical change
B. chemical property
C. physical change
D. physical property
B. chemical property
The number of oxygen (O) atoms that should appear in the product of this reaction.
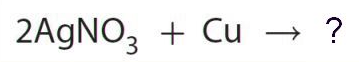
A researcher pours 20.0 mL of each liquid into a test tube. As the two liquids mix, the test tube gets warmer and a yellow solid forms inside. The mass of the yellow solid is 40.0g. 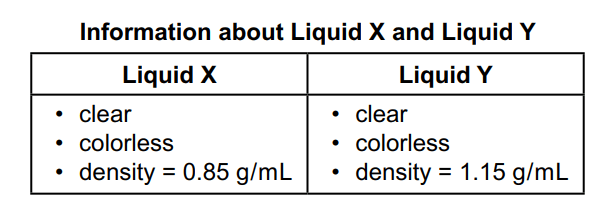
Which statement BEST explains what happened in the test tube?
A. Chem. reaction occurred b/c a new substance was made.
B. Chem. reaction occurred b/c two liquids had different densities.
C. Chem. reaction did not occur b/c a change in state is evidence of only a physical change, not chemical.
D. Chem. reaction did not occur b/c the mass of the liquids before mixing was equal to the mass of the yellow solid.
A. Chem. reaction occurred b/c a new substance was made.
Matter, such as elements and compounds, that cannot vary in composition.
A. substances
B. mixtures
C. reactions
D. curies
A. substances
Which would be the BEST way to separate sand from table salt?
A. use a magnet
B. use a strainer
C. use water
D. use a spoon
C. use water
In which state of matter are there very weak attractions between particles?
A. liquid
B. solid
C. gas
D. clay
C. gas
When a peeled banana turns black, it is a __________.
A. change of state
B. chemical change
C. thermal change
D. physical change
B. chemical change
The part of the atom that contains the protons and neutrons. 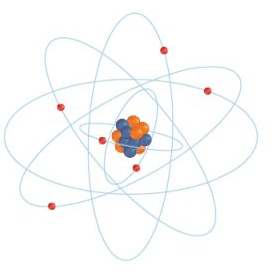
Nucleus
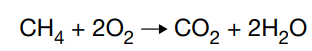
Which statement correctly describes the reactants in the reaction shown?
A. CH4 and O2 are the reactants because they combine to form new compounds.
B. CH4 and O2 are the reactants because they have the highest number of atoms.
C. CO2 and H2O are the reactants because they are formed following an initial reaction.
D. CO2 and H2O are the reactants because they both exist as gases at room temperature.
A. CH4 and O2 are the reactants because they combine to form new compounds.
__________ mixture in which substances are evenly mixed but not chemically bonded, such as Dr. Pepper.
A. clear
B. homogeneous
C. heterogeneous
D. fizzy
B. homogeneous
The following are examples of physical properties except_____.
A. ability to dissolve in water
B. ability to react with oxygen
C. density
D. shape
B. ability to react with oxygen
When a liquid boils, its particles get________. This may be due to the _______in pressure.
A. further apart; decrease
B. closer together; decrease
C. further apart; increase
D. closer together; increase
A. further apart; decrease
When a log burns in a fire,__________.
A. a physical change has occurred
B. mass is gained
C. mass is lost
D. new substances are formed
D. new substances are formed
The number of atoms of hydrogen in each molecule of this compound.
CH4
4
Which of the following is an example of a chemical reaction?
A. lighting a match
B. dissolving salt into water
C. freezing water
D. breaking a glass
A. lighting a match
The number of protons in an atom of an element is the element’s __________.
A. atomic mass
B. chemical symbol
C. atomic number
D. name
C. atomic number
Which is the BEST material to wrap around and insulate an electrical wire?
A. copper
B. silver
C. plastic
D. gold
C. plastic
A change in the state of matter occurs if enough___________is added or removed from an object.
A. thermal energy
B. kinetic energy
C. potential energy
D. force
A. thermal energy
Which of the following is an example of a chemical change?
A. bending a pop can
B. evaporating milk
C. melting wax
D. burning paper
D. burning paper
Sugar is dissolved in water. How does the mass of the solution change?
It will increase by the exact mass of the sugar.
It will stay the same.
It will decrease due to the absorption rate of water.
It will rise by half the mass of the sugar.
1. It will increase by the exact mass of the sugar.
A combustion reaction occurs between one molecule of methane (CH4) and two molecules of oxygen (O2).
How many atoms of carbon, hydrogen, and oxygen make up the products for this combustion reaction?
Carbon: 1
Hydrogen: 4
Oxygen: 4
Examples of this classification of substance, including water, carbon dioxide, and methane, are shown below.
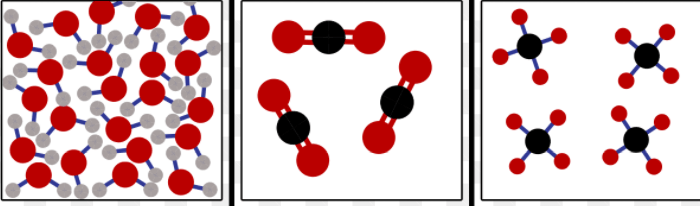
A. elements
B. compounds
C. homogeneous mixtures
D. heterogeneous mixtures
B. compounds
_________ is a physical property.
A. density
B. flammability
C. combustibility
D. oxidation
A. density
Sublimation
Look at the chemical equation.
C3H8 + 5O2 --> 3CO2 + 4H2O
What are the reactants?
C3H8 + 5O2
The number of elements present in this compound. 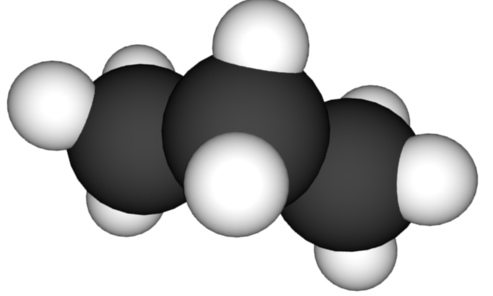
Endothermic reactions________.
A. release more energy than they absorb
B. absorb more energy than they release
C. release and absorb exactly the same amount of energy
D. can either release more energy than they absorb or absorb more energy than they release, depending on the reaction
B. absorb more energy than they release