Physical Properties
1. Type of mixture in which the individual substances are not evenly mixed ____
2. Its composition is always the same.___
1. Heterogeneous mixture
2. Pure Substance (Element or Compound)
What is the formula for Density ?
D = m/v
Which statement best describes the information in this graph?
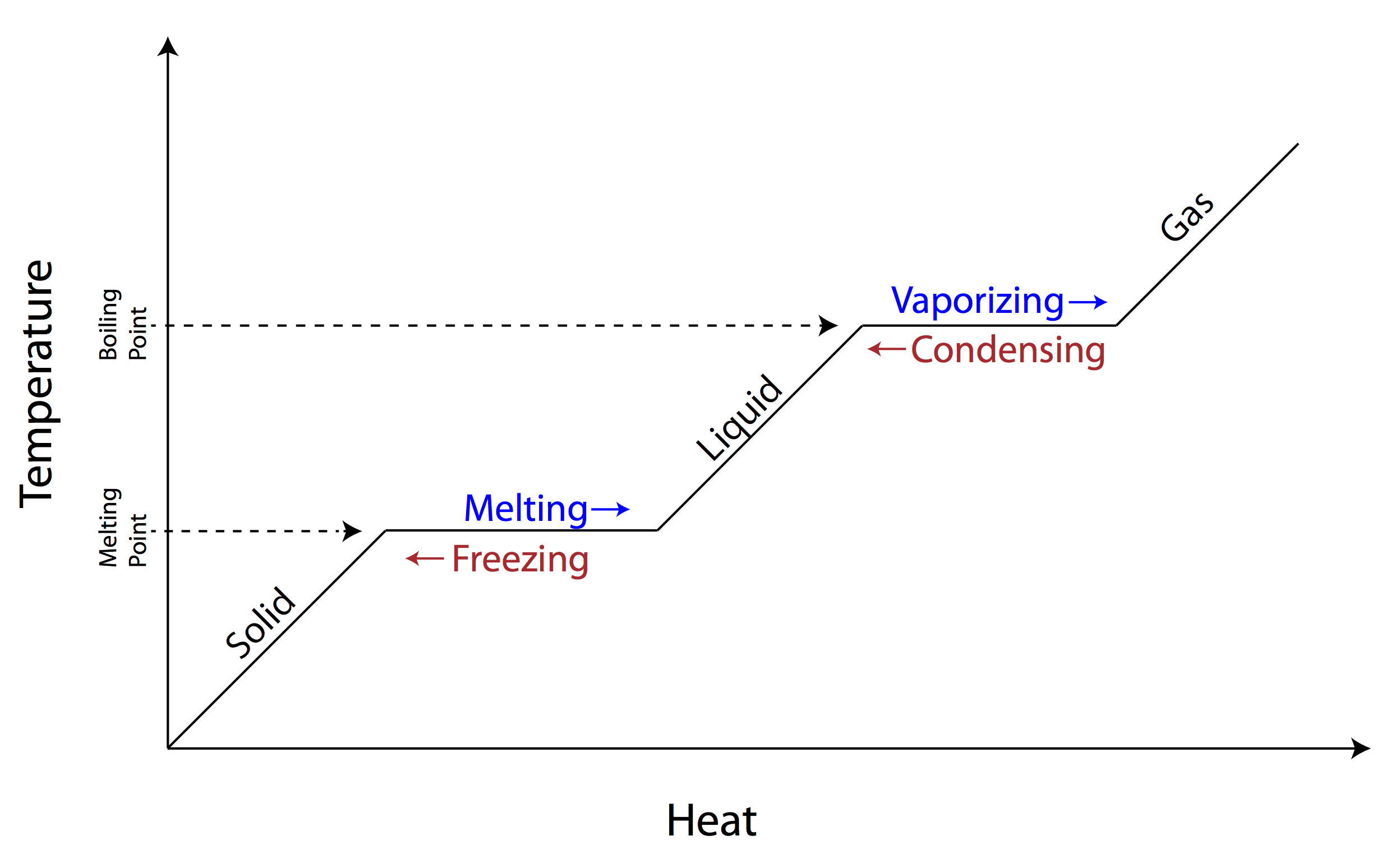
B. As thermal energy increases, temperature also increases and the matter becomes less solid and will change states
How many atoms of hydrogen are in each molecule of this compound?
C6H12O6
12
What are physical methods like boiling and filtering used for when working with mixtures?
To separate them into the various substances that make up the mixture.
1. Matter that can vary in composition
2. Characteristic of matter that you can observe or measure without changing the identity of the matter
1. MIXTURE
2. PHYSICAL PROPERTIES
1. What are the Arrangement of Particles in a
Solid -
Liquid -
Gas -
1. solid--tightly packed, touching, strong attraction, slow moving (vibrating)
liquid--freer moving, more space between with weaking attraction
gas--no attraction, far apart, moving very fast
What happens to a solid if thermal energy is reduced?
particles slow down and come closer together
Matter changing into a substance with new physical and chemical properties is called?
A chemical change/chemical reaction
Feedback: A chemical change results in a new substance with new chemical and physical properties.
Anytime I cook food, the result is what kind of change?
Chemical--you cannot reverse it back to what it was
1. What is a compound?
2. Type of matter in which substances are evenly mixed but not chemically bonded
1. 2 or more elements chemically bonded in a set ratio (has a chemical formula)
2. homogeneous mixture
Describe the physical state of particles in a gas.
no definite shape or volume, always move fast enough and far enough apart to fill their container, little to no attraction to each other
What kind of change occurs if matter changes in amount, size, or shape, but not what the substance is?
physical change
FEEDBACK : Change that does not affect the identity of a matter is known as physical change.
1. Matter changing into a substance with new physical and chemical properties is called?
A. Physical change
B. Elemental change
C. Chemical mixture
D. Chemical change
D Chemical change
Why are both of these models considered elements, instead of compounds?
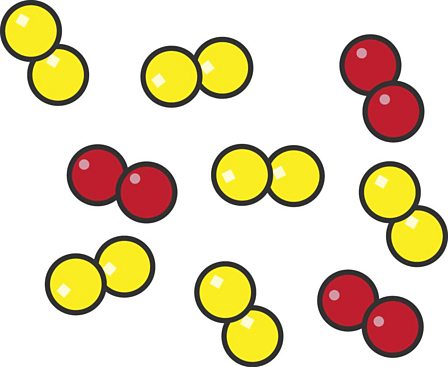
Both have only one kind of element.
1. The number of protons in an atom of an element is the element’s
2. What type of mixture is saltwater?
1. Atomic Number
2. Homogeneous solution
What is the ability of an object to carry heat or electricity?
conductivity
Cara has a sample of saltwater with a mass of 382g. She knows that 94g of salt were added to the solution. What is the mass of the solvent in the solution?
288 Grams
What property does increasing the number of particles of solute in a solution involve?
the concentration
Name 3 size-independent properties of matter
BOILING POINT, melting point, density, state of matter, conductivity, magnetism
Sugar is dissolved in water. How does the mass of the solution change?
A. It will increase by the exact mass of the sugar.
B. It will stay the same.
C. It will decrease due to the absorption rate of water.
D. It will rise by half the mass of the sugar.
A. It will increase by the exact mass of the sugar.
How can the property of magnetism be used to separate a mixture?
A magnet will remove in parts of the mixture that contain iron.
Which conclusion is true about the graph shown here?

Temperature stays the same during a change in state until all particles of the sample have changed state.
The ability to react with other pure substances when introduced to them, is what property of matter? (Hint: it is the opposite of being inert)
Reactivity
If each type of sphere represents a different atom, which statement best describes this model?
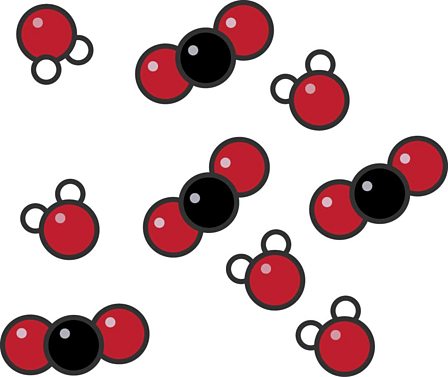
This is a model of a mixture of 2 different compounds, using 3 different elements.
Red element, black element, white element