Which subatomic particles can be found at the nucleus of the atom?
proton, neutron
amu stands for...
isotope
Type of decay where Helium-4 is released.
alpha
What is the charge and approximate amu of:
protons, neutrons, electrons?
p: +1 / 1 amu
n: 0 / 1 amu
e: -1 / 0 amu
Draw two different isotopes of C (can be hypothetical).
p: 6
n: varied
Type of decay that doesn't change the identity of the nucleus. What is it and what does it emit?
gamma, gamma rays
Draw a representation of B with 5 neutrons
n - 5
p - 5
e - 5
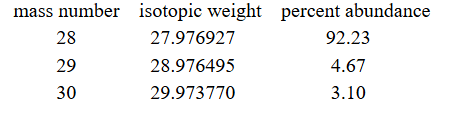
28.086
If you start with 422 mg of calcium-47, how much will remain after 5.00 half-lives have passed?
13.2 mg
How many protons and electrons does the following have:
C
6/6
In a sample of 400 lithium atoms, it is found that 30 atoms are lithium-6 (6.015 amu and 370 atoms are lithium-7 (7.016 amu). Calculate the average atomic mass of lithium.
6.94
Pd-100 has a half-life of 3.6 days. If one had 6.02 x 1023 atoms at the start, how many atoms would be present after 20.0 days?
1.28 x 1022 atoms remain
How many protons and electrons does the following have: P3-
15 / 18
A sample of element X contains 100 atoms with a mass of 12.00 and 10 atoms with a mass of 14.00. Calculate the average atomic mass (in amu) of element X.
12.18
the following is an example of what type of nuclear reaction:

nuclear fission
A particle has 13 protons and 10 electrons. What is its symbolic representation?
Al3+

63.9
Show the following undergoing beta decay:
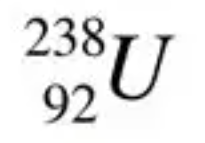
238/92 U -> 0/-1 beta + 238/93 Np