If volume is decreased but temperature stays the same, what happens to pressure?
It increases
What is happening in the following reaction?
2 Ag+ (aq) + 2e- → 2Ag
Reduction
What is 1st law of thermodynamic?
Energy cannot be created nor destroyed, only transformed.
The energy of the universe is constant.
What is Earth's biggest satellite?
The moon
Name two ways that you can increase the pressure of a gas in a bottle.
Heat the bottle (raise temperature) or squeeze bottle (decrease volume)
Name a substance that has an oxidation number of 0.
Any elemental compound.
If a cup of water is our system, what kind of process would freezing water be?
Exothermic
Pikachu is what type of Pokemon?
Electric type
What volume is occupied by 1.00 mol of an ideal gas at 1.00 atm and 0.00C?
22.4 (molar volume)
Identify the reducing agent in the following reaction:
Ag+ (aq) + Cu (s) → Ag (s) + Cu2+ (aq)
Cu (s)
Combustion reactions are what kind of reactions?
Exothermic
Name the movie:
"I Get Emails From A Raccoon, So Nothing Sounds Crazy Anymore.”
Avengers: Endgame
At what temperature (in Kelvin) will an aerosol can go from 2.20atm to 3.00atm at 298K?
406K
What is the oxidation number of chromium in the following compound?
Cr2O72-
ON = 3
True or False: Internal energy is an example of a state function.
True. We only need to know initial and final energy to find internal energy.
Which band is this?
I've become so numb
I can't feel you there
Become so tired
So much more aware
Linkin Park
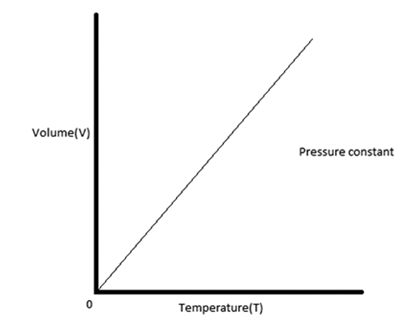
Sketch a volume vs temperature graph representing Charle's law.
Identify the oxidizing agent in the following reaction:
C3H8 + 5O2 → 3CO2 + H2O
O2
Imagine a piston and cylinder in an engine. Fuel combustion produces 155J of energy. Expansion of the gas results in doing 93J of work on the piston. What is the change in internal energy of the system (gas in cylinder)?
Name the song:
"I'm coming out of my cage
And I've been doing just fine
Gotta gotta be down
Because I want it all"
Mr. Brightside