What were the 5 gas laws discussed in class?
Boyle's, Charles's, Avogadro's, Ideal Gas, & Dalton's Law
Convert 2.50 atm to kPa
253 kPa (3 sf)
253.25 kPa
Assume that the temperature and amount of gas remain constant. Solve for the missing variable.
V = 291 mL at 1.07 atm;
V = ? at 2.14 atm
V = 136 mL
What is the ideal gas law formula?
PV = nRT
22.4 L
"The flower that blooms in adversity is the most rare and beautiful of all."
Mulan (1998)/2020/04/29/931/n/1922441/477bc9505ea9efb5e3e429.10894481_PS20_02_28_P.jpg)
In Boyle's law, if the pressure of a gas increases, the volume _________.
decreases (inverse relationship)
Convert 2.99 atm to mmHg
2270 mmHg (3 sf)
2272.4 mmHg
Assume that the pressure and the mass of gas remain constant. Solve for the missing variable.
V = 25 mL a 25 °C
V = ? at 0 °C
V = 0.23 L or 23 mL
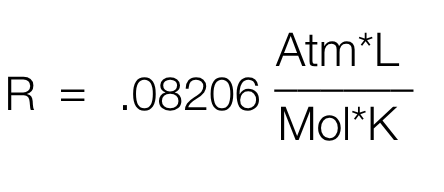
What is the value of R and the units?
Consider the following reaction:
P4(s) + 6H2(g) --> 4PH3(g)
What volume of hydrogen gas, at 298 K and 0.991 atm is required to react exactly with 2.51 g of phosphorus?
“Exercise gives you endorphins. Endorphins make you happy. Happy people just don't shoot their husbands, they just don't.”
Legally Blonde (2001) said by Elle Woods
What is the equation for Charles's Law?
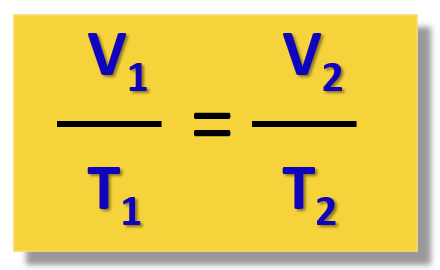
Convert 35℃ to K
308 K
If 2.01 g of helium gas occupies a volume at 12.0 L at 25.0 °C. What volume will 6.52 g of helium gas occupy under the same conditions?
V = 38.9 L
Standard Temperature Pressure
25°C and 1atm
Iron oxide, FeO2, is produced by the reaction:
Fe + O2 → FeO2
How many moles of FeO2 can be produced from 75 L of O2 at STP?
3.3 mol FeO2
"Oh, as if!"

Clueless (1995) said by Cher Horowitz
Which equation uses number of moles (n)?
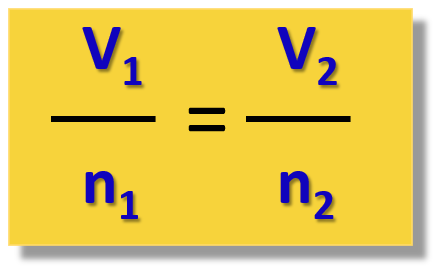
n = number of moles
Convert 250 mL to L
0.250 L
Gas A has a pressure of 0.50 atm, Gas B has a pressure of 1.50 atm, Gas C has a pressure of 2.00 atm. What is the total pressure?
Ptotal = 4.00 atm
(Dalton's Law of Partial Pressures)
Using the ideal gas law, solve for the missing variable. (HINT: CHECK UNITS!)
P = 782.4 mmHg
V = ?
n = 0.1021 mol
T = 299.2 K
V = 2.435 L
What volume does a mixture of 26.2 g of O2 and 35.1 N2 occupy at 35°C and 755 mmHg? (HINT: Use Ideal Gas Law)
V = 52.7 L
"Adventure is out there."

Up (2009) (said by many characters) Ellie Fredrickson
What is the unit of temperature used in gas law calculations?
Kelvin!!
How many pressure units did we go over in this class?
atm, mmHg, torr, kPa, Pa, and psi (6!)
A mixture of four gasses exerts a total pressure of 680 mm Hg. Gasses A and B each exert 220 mm Hg. Gas C exerts 110 mm Hg. What pressure is exerted by gas D?
Gas D Pressure = 130 mm Hg
Using the ideal gas law, calculate for the missing variable. (HINT: CHECK UNITS)
P = 0.998 atm
V = 629 mL
n = ?
T = 35°C
n = 0.0248 mol
An ideal gas has a volume of 50. mL at 100.°C and a pressure of 690. torr. Calculate the volume of this sample of gas at STP. (HINT**Use Combined Gas Law)
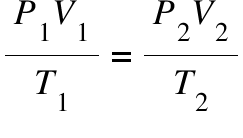
V = .0036 L
"You're a wizard, Harry!"
 Harry Potter and the Socerer's Stone (2001) said by Rubeus Hagrid
Harry Potter and the Socerer's Stone (2001) said by Rubeus Hagrid