What is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3?
This many moles of fluorine are in 3.0 moles of SF4.
What is 12 moles?
If the limits of human hearing are 20 Hz. to 20,000 Hz, state the wavelengths of both values in meters, assuming the speed of sound is 345 m/s.
What is 1.73*101 m and 1.73*10-2 m?
These types of elements do not lose electrons easily and often form anions.
What are nonmetals?
(Freebie) These are the colleges of your respective SI Leaders.
What is college of health sciences and technology and college of science?
This quantum number represents magnetic spin and can take on these values.
What is Ms, +/- 1/2?
Solve the following problem using the appropriate amount of sig figs and scientific notation.
What is the mass of 125 mL gaseous chlorine (density = 3.16 g/L)?
What is 0.395 g?
This color has the highest visible wavelength range.
Ionic compounds are made up of these two ions of opposing charges.
What are cations and anions?
This is the actual length of the quarter mile in kilometers. (Fun fact it's actually a third of a mile:))
What is 5.36*10^-1 km?
These are the two elements with exceptions to the typical rules of electron configuration that you will most likely be tested on. How do they violate it?
What are chromium and copper? They leave the 3d subshell half (Cr) or completely (Cu) full so they are more stable.
Solve the following.

What is 1.20x10^23 molecules?
This is the wavelength range of Ellen's favorite color.
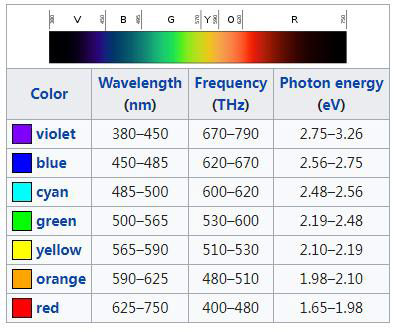
What is 565-590?
Define ionization energy.
What is the amount of energy required to remove an electron from an atom?
What is the periodic trend for effective nuclear charge?
What is decreases down a group and increases across a period OR increases diagonally going up and right?
Draw the orbital diagram for a silicon electron.
What is 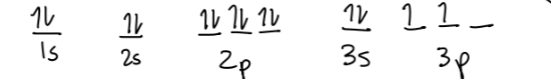 ?
?
Gianna has 8.2 moles of carbon and 8.2 moles of helium. She has more grams of this element.
What is carbon?
This is the frequency of a yellow light given off by a sodium vapor lamp used for public lighting with a wavelength of 589 nm.
What is 5.09*1014 Hz?
Write the condensed formula of the following compound (butane).
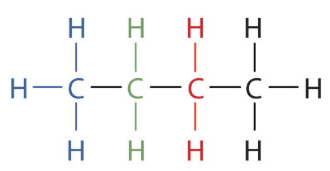
What is CH3CH2CH2CH3?
This element has 15 protons, 16 neutrons, and 17 electrons. State the charge and atomic number of the element.
What is -2 and 15?
I am an electron. I have jumped from the full orbital of 3d to 5p. This is the light-related process that I have just executed.
What is light absorption?
Ellen has 4.5 mg of nitrogen gas. This is the amount of moles of nitrogen she has in scientific notation.
What is 1.6*10-4 mol N2?
Microwave ovens emit microwave energy with a wavelength of 12.9 cm. This is the energy of exactly one photon of this microwave radiation.
What is 1.54*10-24 J?
Write the basic chemical formula for the following compound (diethyl ether).
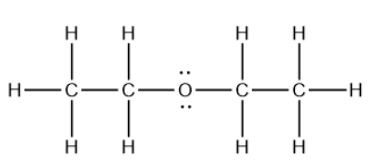
What is (CH3CH2)2O?
This element has 25 protons, 30 neutrons, and 23 electrons. State the mass number of this element.
What is 55?