5.67 cm + 12.3 cm + 4.55 cm = ?
22.5 cm
Write the formula and the name of the ionic compound formed between barium (Ba) and phosphorus (P)

Ba₃P₂
Barium phosphide
Determine the Lewis structure and the name of the compound formed between boron (B) and chlorine (Cl)

Boron trichloride
Balance: Fe + O₂ → Fe₂O₃
4Fe + 3O₂ → 2Fe₂O₃
Name the three transition metals that only appear in one cationic form and the charges they have
Zinc (Zn2+), cadmium (Cd2+), and silver (Ag+)
125.678 m – 0.002 m – 1.2 m = ?
124.5 m (tenths place)
Write the formula and the name of the ionic compound formed between iron(II) and sulfate

FeSO₄
Iron(II) sulfate
Determine the electron and molecular geometry of the central carbon in carbon dioxide

Electron geometry: linear
Molecular geometry: linear
In 4Al + 3O₂ → 2Al₂O₃, how many moles of Al₂O₃ are produced per 4 mol Al?
2 mol Al₂O₃
Under what conditions can hydrogen bonding occur?
Hydrogen atom bonded to F, O, or N atom is attracted to a lone pair on a different F, O, or N atom
6.022×10^23 mol⁻¹ × 2.0 mol = ?
1.2 × 10²⁴ (2 sig figs)
Name the ionic compound SnF₄

Tin(IV) fluoride
Determine the electron and molecular geometry of the nitrogen in nitrogen trihydride

Electron geometry: tetrahedral
Molecular geometry: trigonal pyramidal
In the reaction Zn + Cu²⁺ → Zn²⁺ + Cu, which element is oxidized and which is reduced?
Zn is oxidized (from 0 to 2+, is losing electrons)
Cu is reduced (from 2+ to 0, is gaining electrons)
Define oxidation and reduction
Oxidation occurs when something loses electrons
Reduction occurs when something gains electrons
OIL RIG
0.0550 kg ÷ 0.50 s = ?
0.11 kg/s (2 sig figs)
Name the ionic compound Pb(NO₂)₂

Lead(II) nitrite
Determine the types of intermolecular forces that nitrogen trihydride would participate in.
Dispersion forces, dipole-dipole forces, hydrogen bonding, and ion-dipole if an ion was present
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O.
How many grams of CO₂ are produced from 44.0 g C₃H₈? (1 mol C₃H₈ = 44.0 g)

132 g CO₂
Combustion reactions are associated with the production of
Carbon dioxide (CO2) and water (H2O)
5.00×10^1 J + 4.5 J = ?
5.45 ×10^1 J
Write the formula and the name of the ionic compound formed between ammonium and carbonate

(NH₄)₂CO₃
Ammonium carbonate
Draw the Lewis structure of CH3OH, then determine what types of intermolecular forces this molecule would participate in.

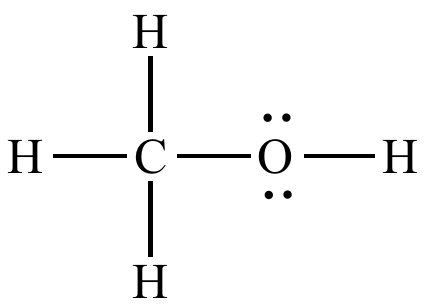
Dispersion forces, dipole-dipole interactions, hydrogen bonding, and ion-dipole if an ion was present
C4H10+ 13O2 → 8CO2 + 10H2O
How many grams of water are produced when 20.0 g of butane (C4H10) is burned completely in oxygen?

62.0 g H2O
Solving:
20.0 g C4H10 = 0.334 mol C4H10
0.334 mol C4H10 x (10 mol H2O / 1 mol C4H10) = 3.44 mol H2O
3.44 mol H2O × 18.02 g/mol = 62.0 g H2O
What score is everyone going to get on the exam?
100!