What is the strongest electrolyte in dilute aqueous solution?
A) HClO4
B) HCN
C) HF
D) HNO2
A) HClO4
All gases approach ideal behavior under conditions of
A) low density and high temperature
B) low density and low temperature
C) high density and high temperature
D) high density and low temperature
A) low density and high temperature
Give the name for FeBr3
Iron (III) Bromide
The limiting reagent for a given reaction can be recognized because it is the reagent that
A) has the smallest coefficient in the balanced equation for the reaction.
B) has the smallest mass in the reaction mixture.
C) is present in the smallest molar quantity.
D) would be used up first.
D) would be used up first
How many covalent bonds are represented in the formula NH4Cl?
What is the molar concentration of the chloride ion in a 3.0 M CaCl2 solution?
6.0 M
Suppose a gas mixture contains equal moles of He(g) and O2(g). Which is true?
A) The partial pressure of each gas is the same.
B) The partial pressure of He(g) is four times the partial pressure of O2(g).
C) The partial pressure of O2(g) is two times the partial pressure of He(g).
D) The partial pressure of O2(g) is eight times the partial pressure of He(g).
A) The partial pressure of each gas is the same.
How many moles of hydrogen atoms are in six moles of Ca(OH)2?
12
What are the smallest whole number coefficients for each substance (respectively) in the chemical reaction when balanced?
_NaBr + _Cl2 --> _NaCl + _Br2
A) 1,1,2,2
B) 1,2,2,2
C) 2,1,2,1
D) 2,4,2,4
Which molecule contains carbon with a negative formal charge?
A) CO
B) CO2
C) H2CO
D) CH4
A) CO
What is the molar concentration of a solution when 7.00 mL of a 3.25 M aqueous solution is diluted to 25.00 mL?
0.910 M
According to kinetic-molecular theory, why does pressure increase as the temperature of an ideal gas increases?
I. The gas molecules collide more frequently with the wall.
II. The gas molecules collide more energetically with the wall.
A) Only I
B) Only II
C) Both I and II
D) Neither I nor II
C) Both I and II
If gallium, atomic number 31, combines with selenium, atomic number 34, what is the most likely formula based on your knowledge of the periodic nature of the elements?
Ga2Se3
How many moles of water are necessary to react completely with 8.0 mol PCl5?
PCl5 + 4H2O --> H3PO4 + 5HCl
32 moles
A central atom has two lone pairs and three single bonds. What is the molecular geometry?
T-shaped
Which combination will result in a precipitate forming?
A) NaOH(aq) and HCl(aq)
B) NaOH(aq) and FeCl3(aq)
C) NaNO3(aq) and FeCl3(aq)
D) Zn(s) and HCl(aq)
B) NaOH(aq) and FeCl3(aq)
A sample of gas occupies 3.00 L at 1.00 atm. What volume will it occupy at 1.45 atm and the same temperature?
2.07 L
A molecular compound is found to consist of 30.4% nitrogen and 69.6% oxygen. If the molecule contains 2 atoms of nitrogen, what is the molar mass of the molecule?
92 g/mol
What amount of Al2O3 is produced from the reaction of 3.0 mol Al with 2.0 mol Fe2O3?
2Al + Fe2O3 --> Al2O3 + 2Fe
1.5 mole
Which molecule has 1200 bond angles?
A) CO2
B) NH3
C) IF3
D) SO3
D) SO3
What particles would be present in NH3(aq)?
A) water molecules, NH4+ ions, and OH- ions
B) water molecules, H3O+ ions and NH2- ions
C) water molecules and NH3 molecules
D) water molecules and NH3 molecules and a small number of NH4+ ions and OH- ions
D) water molecules and NH3 molecules and a small number of NH4+ ions and OH- ions
A 3.41 x 10-6 g sample is known to contain 4.67 x 1016 molecules. What is this compound?
A) CH4
B) CO2
C) H2O
D) NH3
B) CO2
What amount of the excess reagent remains when 0.500 mol Li reacts with 0.350 mol N2?
6Li (s) + N2 (g) --> 2Li3N (s)
0.267 mol N2
How many sigma and pi bonds are in the image below? 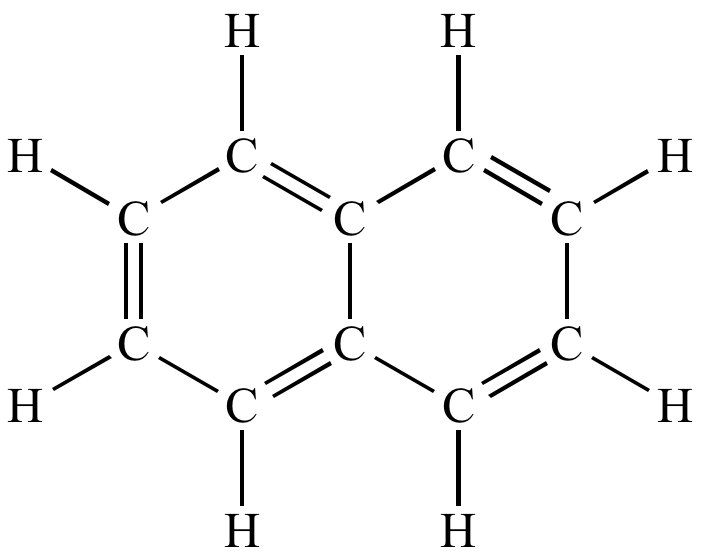
19 sigma and 5 pi