If a wavelength is short, is the corresponding frequency high or low?
High
What are the four quantum numbers?
n, l, ml & ms
What is the most electronegative?
Fluorine
What does the electron/molecular geometry tell us that a lewis structure cannot?
It can tell us what the molecule looks like in 3D
How many bonds does Hydrogen make?
ONE
Would a photon with low Energy have a long or short wavelength?
Long
If l = 1, what values can ml have?
ml = -1, 0, +1
How many valence electrons will be used in the Lewis Structure of SO42-?
32 e-
What type of electron geometry does CO2 have?
Linear
What is the conversion factor for nm to m
1m = 109nm
What type of electromagnetic radiation has the longest wavelength?
Radio Waves
Which quantum number is not correct?
n = 3, l = 1, ml = -2, ms = +1/2
ml Can not be -2
Arrange the elements (K, Ne, Ar, Na and P) in order from smallest to largest
radii?
Ne<Ar<P<Na<K
What Electron geometry does BF3 have?
Trigonal Planar
After which element can there be an expanded octet?
After Magnesium
Which type of electromagnetic radiation has the highest frequency?
Gamma Radiation
What is the Noble Gas electron diagram configuration for Fe?
[Ar] 4s2 3d6
Draw the Lewis structure for PCl5
What Molecular geometry does H2O have?
Bent
What is a covalent bond made of?
Two non-metals
What is the frequency of the yellow-orange light (λ=589 nm) produced by
sodium-vapor streetlights?
5.09 x 1014Hz
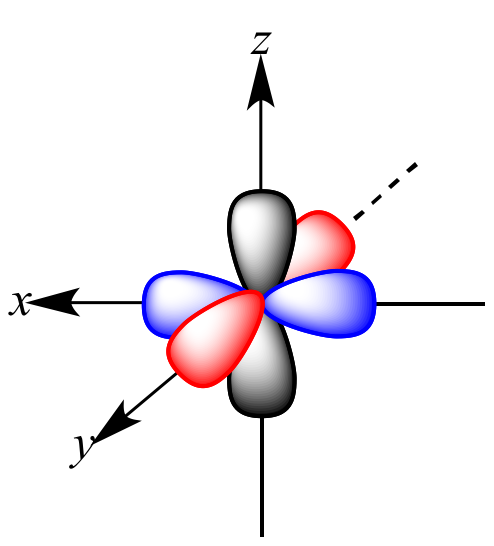
What does a "p" orbital look like?
Draw the lewis structure for SO42-
What is the Electron and Molecular geometry of NH3?
Tetrahedral and Trigonal Pyramidal
Is CO2 polar or non-polar?
Non-polar