What is the mass of one mole of Tin?
119g
Draw the Lewis Structure for Neon
Melting an ice cube
Physical Change
Find the volume of a box with a length of 5.00 cm, width of 10.0 cm, and a height of 2.00 cm.
100. cm3 (5cm x 10 cm x 2 cm)
Significant Figures show how _____________ a measurement is.
Accurate
What is the mass of one mole of Carbon monoxide?
28.0 g
Draw the Lewis Structure for Carbon tetrabromide

Is Adding vinegar to baking soda and seeing bubbles a chemical or physical change.
Chemical Change
Calculate the volume of a rubber stopper if the water in a graduated cylinder rose from 50.00 mL to 61.53 mL after the stopper was submerged.
11.53 mL
The number of significant figures in 4.2400 x 10-7 m
5(non-zeros + trailing zeros after a decimal)
What is the mass of one mole of FeCl2
162g
Draw the Lewis Structure for hydrogen cyanide.
H C N
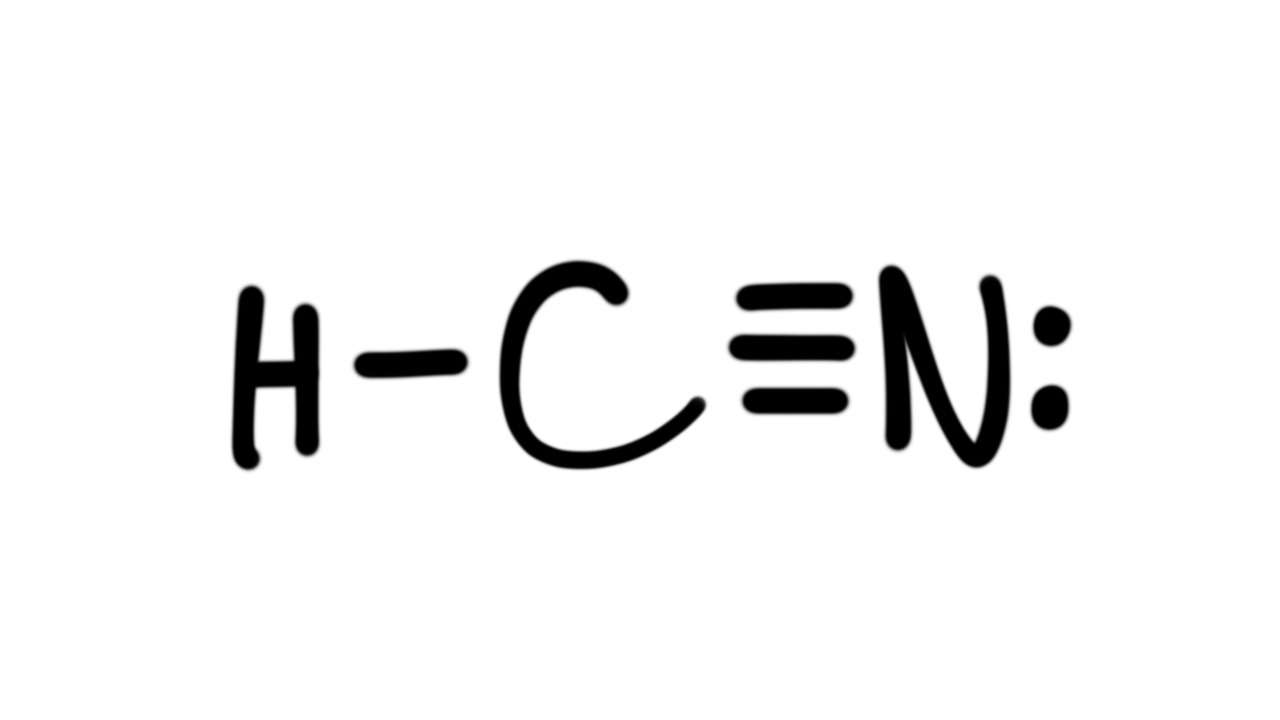
Sodium added to water and catching on fire
Chemical Change
Find the density of an object with a volume of 93.5403 cm3 and a mass of 42.8 g.
D= 0.458 g/cm3
The number of zeros that are significant in 30.02000 mm
5 (all trapped zeros + all trailing zeros after a decimal)
What is the mass of
5.67 moles of LiNO3
391g
Draw the Lewis Structure for
H H
H C C O H
H H

Dissolving sugar in ice tea
Physical Change
What is the mass of a stone with a volume of 25.64 cm3 and a Density of 9.65 g/cm3
247 g
Convert 103.20 L to scientific notation. Keep the same number of significant figures.
1.0320 x 102 L
What is the mass of 3.54 moles of
Sodium Chloride
207 g
Draw the Lewis Structure for SO3 -2
Baking a Cake
Both chemical and physical change
What is the volume of an object with a Density of 2.635 g/mL and a mass of 6.29 g?
2.39 mL
Convert 6 000. m to scientific notation. Keep the same number of significant figures.
6.000 x 103 m