Seeing this in a scientific notation would tell us that the number in standard notation is less than the value of 1.
What is a negative exponent?
These zeroes in a number don't count toward the significant figure value regardless of whether there is a decimal in the number.
What are leading zeroes?
The number of centimeters (cm) in a meter.
What is 100?
This would be the density of 180 g of helium occupying 11000 mL.
What is 0.016 g/mL?
This is the order of the states of matter from MOST to LEAST dense.
What is solid, liquid, then gas?
The value of the number written in front of the "x 10x " must always be between . . .
What is 1 and 9.99?
The number 56790 rounded to three significant figures would be. . .
What is 56800?
56000 grams (g) expressed as kilograms (kg).
(1 g = 0.001 kg)
What is 56 kg?
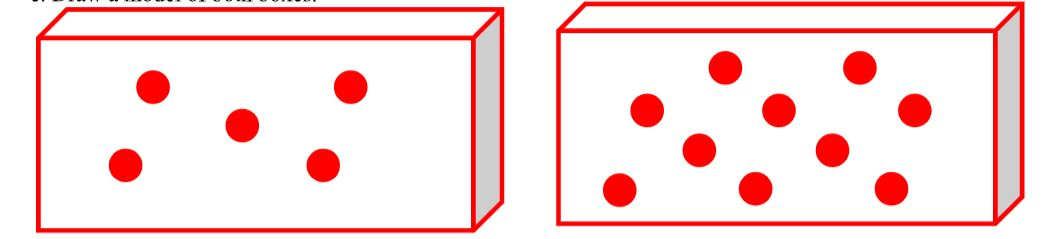
Of the shown boxes with the same volume, this box has the lower density.
What is box A (left)?
milliliters (mL), liters (L), gallons (gal), cubic centimeters (cm3) are all units for this form of measurement.
What is volume?
5.00 x 103 written in standard notation.
What is 5000?
Which of the following numbers has a zero that is NOT a significant figure?
A. 490.05
B. 11020.0
C. 0.004883
D. 9044
Option C
The numerical value of millimeters and kilometers that is equivalent to each other.
What is 0.001 km = 1000 mm?
There is no doubt that there was room for Jack on the wooden door from Titanic. Rose was just being selfish. Which of the following seems a probable density for wood?
A. 0.65 g/cm3, the density should be less than water's because wood floats
B. 10.8 g/cm3, the density should be less than water's because wood floats
C. 0.65 g/cm3, the density should be greater than water's because wood floats
D. 10.8 g/cm3, the density should be greater than water's because wood floats
What is option A?
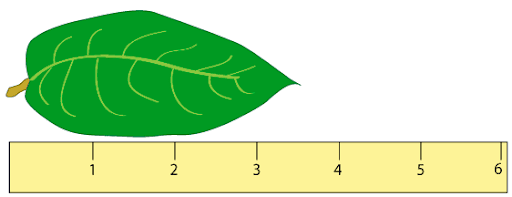
The measurement of the leaf shown above in the correct number of significant figures.
What is 3.6 cm? (2 significant figures)
7.9 x 10-3 written in standard notation.
What is 0.0079?
The number of significant figures in 0.01060700 (list which ones are significant)
What is seven?
The two leading zeroes don't count. Everything else does.
If a car has a tank that can fill 13.1 gallons (gal) of gas, this is how many centiliters (cL) that would be.
(1 gal = 3.79 L, 100 cL = 1 L)
What is 4964.9 centiliters (cL)?
This the volume of a 451.00 g block of aluminum whose density is 2.7 g/cm3
What is 167.04 cm3?
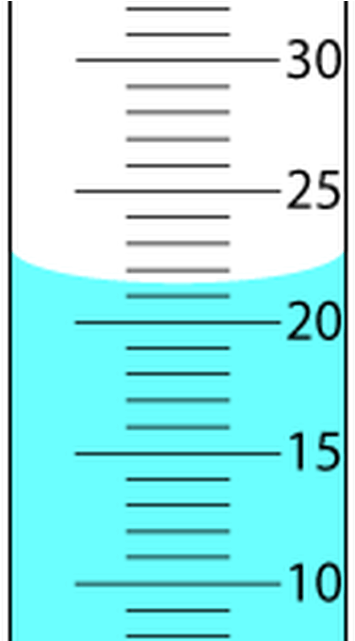
The measurement shown above
What is 21.5 mL? (3 sig figs)
0.000657 written in scientific notation.
What is 6.57 x 10-4?
The number 0.01060700 rounded to two significant figures would be. . .
What is 0.011?
If someone is 5.5 feet tall, this would be their height in millimeters.
(1 foot = 12 inches, 1 inch = 2.54 cm, 100 cm = 1000 mm)
What is 1676.4 mm?
A sample of gold has a density of 19.3 g/cm3 and a volume of 77.77 cm3. A sample of copper has the same mass as the sample of gold except with a density of 8.96 g/cm3.
What is the volume of the copper?
What is 167.52 cm3?
(Daily Double!!)
The actual density of ice is 0.92 g/cm3. In a lab, you find the mass of an ice cube is 40 g and it occupies a volume of 40 cm3. What was your percent error?
8.70 percent