He proved the Law of Multiple Proportions.
A. Antoine Lavoisier
B. Joseph Louis Proust
C. John Dalton
C. John Dalton
He's an English chemist who introduced the law of multiple proportions stating that when two elements combine to produce one or more compounds, the ratio of the masses of an element given a fixed mass of the other element, is of small, whole numbers.
TRUE OR FALSE
Dalton adapted the belief of Democritus and Leucippus that atoms are tiny, indivisible units making up the matter.
TRUE
What is the negatively-charged subatomic particle?
ELECTRON
What is the isotope of carbon used to date materials from living organisms?
A. carbon-12
B. carbon-13
C. carbon-14
C. carbon-14
What is the alternative way to illustrate structural formulas?
ANSWER: MOLECULAR MODELS
The discovery of oxygen and its necessity in combustion leads us to the idea that ___________.
A. matter is neither created nor destroyed
B. matter is composed of tiny, indivisible particles called atoms
C. a compound is composed of different elements
A. matter is neither created nor destroyed
TRUE OR FALSE
Compounds with the formula NO and N2O pertain to the same substance since they are both made up of nitrogen and oxygen.
FALSE
A substance is a material that has a definite composition and set of properties.
Which of the following is true about isotopes?
a. the same number of protons and neutrons
b. the same number of neutrons but a different number of protons
c. the same numbers of protons but a different number of neutrons
d. the same mass number
c. the same numbers of protons but a different number of neutrons
Which isotope of iodine is used in thyroid imaging?
A. iodine-123
B. iodine-127
C. iodine-131
A. iodine-123
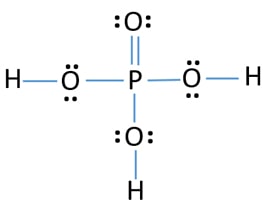
Identify the chemical formula corresponding to the following structural formula above.
A. H2PO3
B. H3PO3
C. H3PO4
D. H2P2O4
C. H3PO4
In a process, 24g of carbon reacts with hydrogen to produce three compounds wherein the masses of hydrogen are 2g, 4g, and 8g. What basic law of matter is presented?
A. law of conservation of mass
B. law of definite proportion
C. law of multiple proportion
C. law of multiple proportions
The ratio of the mass of oxygen to the mass of hydrogen is presented in three proportions.
24:2 or 12:1
24:4 or 6:1
24:8 or 3:1
TRUE OR FALSE
Different samples of the same compound have the same relative proportions of elements present.
TRUE
A compound is made up of a combination of different kinds of atoms following a specific ratio according to Dalton's atomic theory.
How many protons are there in carbon-14 (Z=6)?
Carbon-14 has six (6) protons.
14 - mass number
6 - atomic number
Upon administration of a compound containing this isotope, it is absorbed into the bloodstream which then proceeds to the thyroid gland. At this point, the isotope kills the cells of the thyroid gland. What isotope was used in the process?
IODINE-131
What is the empirical formula of C6H10O4?
A. C2H5O2
B. C3H6O1
C. C3H5O2
C. C3H5O2
Heating of baking soda results in decomposition into lye and carbon dioxide. Once the baking soda is used up, its mass must be equal to the masses of lye and carbon dioxide. What basic law of matter is presented?
Law of Conservation of Mass
What is the 4th statement of Dalton's Atomic Theory?
During a chemical reaction, atoms combine, separate, or rearrange. No atoms are created and no atoms disappear.
An atom is neither created nor destroyed.
How many protons, electrons, and neutrons, respectively, are there in lead 206 (Z=82)?
ANSWER: 82, 82, 124
82 - atomic number
82 - no. of electrons
124 - no. of neutrons (206-82=124)
What is the use of Cobalt-60 which emit gamma rays?
It can be used in the sterilization of food products which kills the bacteria without affecting the quality of the product. It can be used in the treatment of cancer.
What is the molecular formula of CH2 if n=4?
Molecular Formula = (Empirical Formula)n
MF = (CH2)4 = C4H8
If there are 20g of oxygen in a sample of Aluminum Oxide (Al2O3). How many grams of aluminum are present? Show your complete solution.
For every 20g of oxygen, there is 22.4g of aluminum.
20g x (54g/48g) = 22.4g
All properties of carbon dioxide (CO2) and carbon disulfide (CS2) are the same. What statement of Dalton's Atomic Theory contradicts this idea?
The 2nd statement states that every element is made up of one kind of atom and that it has a different size, properties, and mass from each other.
What atomic model describes an atom as a positively-charged cloud with scattered electrons?
PLUM-PUDDING MODEL
Why isotopes are said to be radioactive?
ANSWER: They are radioactive as they emit small ions and high-energy radiation.
Write the two (2) disadvantages of the ball-and-stick model.
ANSWER:
1. balls used are not proportional (actual size of the atoms)
2. sticks used may not represent the appropriate bond length between atoms