The conversion factors from meters to centimeters are:
Solve for z:
4z/-12=-8
24
730 in scientific notation
7.30x10^2
Identify the larger number
I. 7.2×10^4 and 8.2×10^3
II. 0.00052 and 5.4×10-5
III. 12500 and 3.4×10^4
I. 8.2x10^3
.00052
3.4x10^4
Write the following in scientific notation
.000713
7.13x10-4
A length of glass tubing is 0.525 m. How many inches long is the tubing? (2.54 cm = 1 inch)
20.7 in
Because high fevers can cause convulsions in children, the doctor needs to be called if the child’s temperature goes over 40.0 °C. Should the doctor be called if a child has a temperature of 103 °F?
No. The temperature is equivalent to 39 °C.
Convert 59873300 nm to km
5.98733e-5
1. If you smell, see bubbles, or fizz is this a chemical or physical change
Chemical change
Which is a pure substance?
1. O2
2. CO2
3. O2 + N2
1 and 2
A child who fell through the ice on a lake has hypothermia with a core temperature of 32 °C. What is this temperature in degrees Fahrenheit?
90 F
The element tungsten used in light bulb filaments has a density of 19.3g/cm^3. What is the volume, in cubic centimeters, of 250 g of tungsten?
13cm^3
heat 25.0 g of water from 12.5 °C to 25.7 °C
specific heat is 1cal per gram per celsius degree
1380 J, 330. cal
Ignore the cm.. what is this measurement in mm
6.500
Classify each of the following as a pure substance or a mixture:
baking soda
a blueberry muffin
ice
zinc (Zn)
trimix (oxygen, nitrogen, and helium) in a scuba tank
b.mixture
c.pure substance
d.pure substance
e. mixture
The price of 1 lb of potatoes is $1.75. If all the potatoes sold today at the store bring in $1420, how many kilograms of potatoes did grocery shoppers buy?
368 kg
What is the density (g/mL) of a sample of mineral oil if 250 mL has a mass of 0.23 kg?
.92 g/mL
3.27 The energy needed to keep a 75-watt light bulb burning for 1.0 h is 270 kJ. Calculate the energy required to keep the light bulb burning for 3.0 h in each of the following energy units:
joules
kilocalories
8.1x10^5 J
- 190 kcal
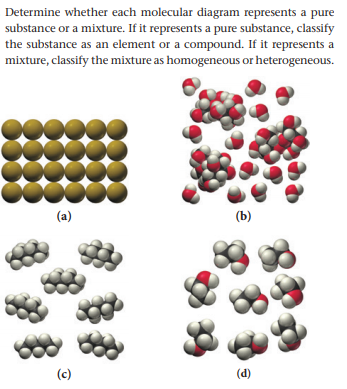
Determine if mixture or pure substance
1. pure substance, element
b. mixure
c. pure substance, compound
d. pure substance, compound
Describe each of the following properties for the element fluorine as physical or chemical:
is highly reactive
is a gas at room temperature
has a pale, yellow color
will explode in the presence of hydrogen
has a melting point of -220C
chemical
physical
physical
chemical
physical
During a workout at the gym, you set the treadmill at a pace of 55.0 m/min. How many minutes will you walk if you cover a distance of 7500 ft?
1foot=.3048
42 minutes
To treat a bacterial infection, a doctor orders 4 tablets of amoxicillin per day for 10 days. If each tablet contains 250 mg of amoxicillin, how many ounces of the medication are given in 10 days?
1oz=28.3405grams
0.35 oz
The melting point of benzene is 5.5 °C and its boiling point is 80.1 °C. Sketch a heating curve for benzene from 0 °C to 100 °C.
What is the state of benzene at 15 °C?
What happens on the curve at 5.5 °C?
...
Cabohydrate = 4 kcal/g
Fat = 9 kcal/g
Protein = 4 kcal/g
A tablespoon of peanut butter contains 5.0 g of fat but and 8.0 grams of protein. What is the total amount of nutritional Calories (Cal) obtained by a tablespoon of
peanut butter?
77 Cal
Substances A and B are the same mass and at the same initial temperature. When they are heated, the final temperature of A is 55 °C higher than the temperature of B. What does this tell you about the specific heats of A and B?
The specific heat of A must be less than the specific heat of B
