 The number 2 is Helium's ____________. Which directly correlates to the amount of ________ in the nucleus of the atom.
The number 2 is Helium's ____________. Which directly correlates to the amount of ________ in the nucleus of the atom.
a. Atomic number; protons
b. Atomic number; neutrons
c. Atomic mass; electrons
d. Atomic mass; circles
a. Atomic number; protons
How many of the following particles are shown in this image?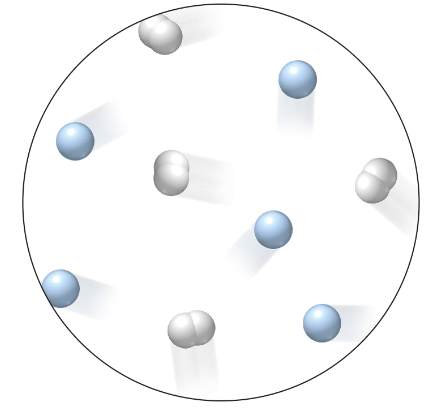
- Hydrogen atoms
- Hydrogen molecules
- Helium atoms
8 hydrogen atoms
4 hydrogen molecules
5 helium atoms
How many grams are in 1 kg?
1000g
what is the exponential value if the prefix is deci?
10-1
 The number 4.0026 represents the _________ of Helium.
The number 4.0026 represents the _________ of Helium.
Average atomic mass
Which of the following statements best describes the change depicted in the figure:
- A mixture of two gaseous elements undergoes a chemical reaction, forming a gaseous compound.
- A mixture of two gaseous elements undergoes a chemical reaction, forming a solid compound.
- A mixture of two gaseous elements undergoes deposition.
- A mixture of two gaseous elements condenses.
2. A mixture of two gaseous elements undergoes a chemical reaction, forming a solid compound.
Fill in the blank:
Always convert through the _______ _______.
base unit
What is the exponential value if the prefix is kilo?
103
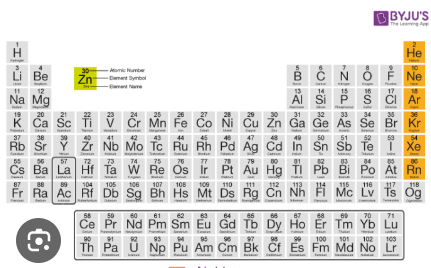 What is the name of the group of elements highlighted in orange?
What is the name of the group of elements highlighted in orange?
Noble Gases
Which of the following properties of water are chemical and which are physical?
a. Water normally freezes at 0.0℃.
b. A cork floats on water, but a piece of copper sinks.
c. During digestion, starch reacts with water to form sugar.
Chemical: C
Physical: A and B
What are the SI units for the following:
a. length
b. volume
c. mass
d. time
e. temperature
a. meter (m)
b. meter3
c. kilogram (kg)
d. second (s)
e. kelvin (K)
What is the exponential value if the prefix is centi?
10-2
How can we determine the number of neutrons in the nucleus of an element just from looking at the periodic table?
Subtract the atomic number from the average atomic mass.
Which of the following statements best describes the change depicted in the firgure? 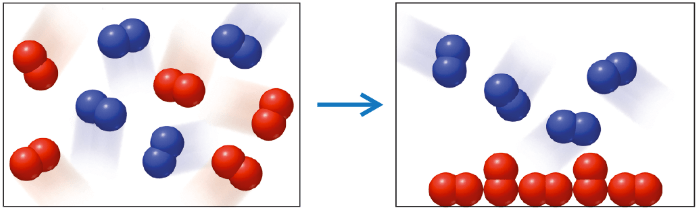
- A mixture of two gaseous elements is cooled to a temperature at which one of them condenses.
- A mixture of two gaseous compounds is heated to a temperature at which one of them decomposes.
- A mixture of two gaseous elements undergoes deposition.
- A mixture of two gaseous elements reacts together to form two compounds, one of which is a liquid.
1. A mixture of two gaseous elements is cooled to a temperature at which one of them condenses.
How many meters are in 12 inches?
1 inch = 2.54 cm
0.3048 m
What is the exponential value if the prefix is micro?
10-6
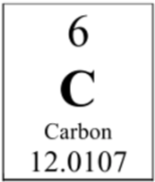
How many neutrons are in the nucleus of a carbon atom?
6 neutrons
For each image, identify what class of matter is depicted (an element, a compound, a homogeneous mixture, or a heterogeneous mixture) and identify the physical state(s).
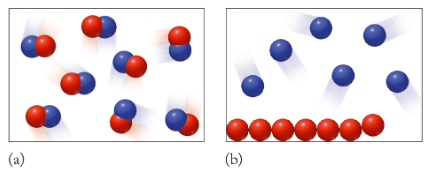
(a) A pure compound in the gas phase
(b) a heterogeneous mixture of blue-element atoms and red-element atoms: blue atoms are in the gas phase and red atoms are in the liquid phase.
Convert 3.25 x 106 cm into kilometers.
32.5km
What is the exponential value if the prefix is femto?
10-15