Which choice correctly identifies anabolism and catabolism with their corresponding pathways? (LO 1)
A. Anabolism: using ATP to build a large molecule, gluconeogenesis; Catabolism: using fuel to make ATP, glycolysis
B. Anabolism: using fuel to make ATP, gluconeogenesis ; Catabolism: using ATP to build a large molecule, glycolysis
C. Catabolism: using ATP to build a large molecule, glycolysis; Anabolism: using fuel to make ATP, gluconeogenesis
D. Catabolism: using fuel to make ATP, gluconeogenesis ; Anabolism: using ATP to build a large molecule, glycolysis
A. Anabolism: using ATP to build a large molecule, gluconeogenesis; Catabolism: using fuel to make ATP, glycolysis. (Glycolysis case study intro PowerPoint slide 31)
What causes 1,3-bisphosphoglycerate and phosphoenolpyruvate to undergo this type of reaction at Step 7 and Step 10? (LO 9)
1,3-bisphosphoglycerate (1,3-BPG) and phosphoenolpyruvate (PEP) have higher phosphoryl transfer potential than ATP. These substrates undergo substrate-level phosphorylation. (slide 16 of Glycolysis Case Discussion).
After a patient's PET scan, it was observed that there were abnormal cells based on the results. What type of glycolysis is taking place if the cells were taking in a lot of FDG and producing a lot of lactate? Use the Warburg effect in the answer. (LO 11)
Aerobic glycolysis.
This pertains to the Warburg effect since cancer cells or proliferated tissues tend to go through aerobic glycolysis even in the presence of oxygen and this can be observed in the question. (Glycolysis Case Introduction Slide 46, Glycolysis Case Study Question 11)
What molecules are being oxidized and reduced in cellular respiration and why? Include electron carriers in your explanation. (LO 2)(Image Citation: Khan Academy)
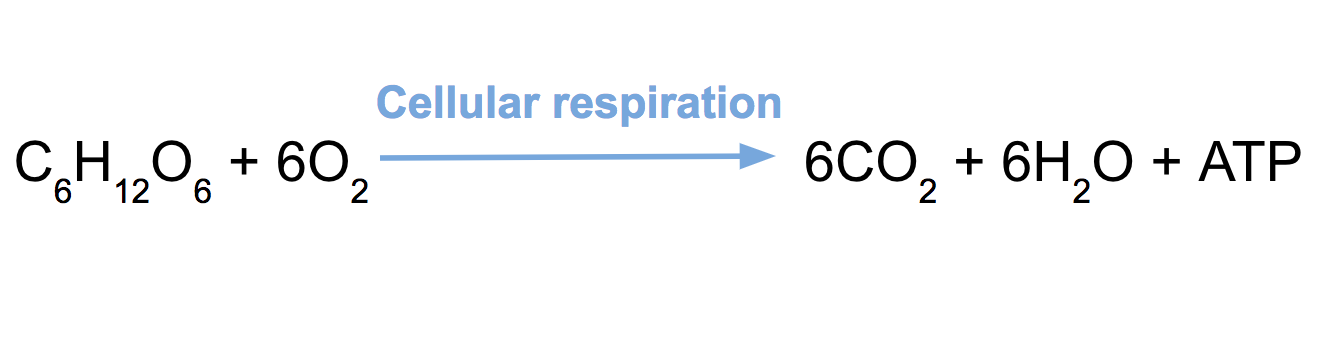
Glucose is being oxidized into carbon dioxide. Oxygen is being reduced into water. The electron carriers are NAD and FAD within cellular respiration. (In-class Glycolysis Lecture and Glycolysis Case Introduction slide 19-20)
What are the factors that contribute to negative G of hydrolysis (related to the phosphoryl transfer of ATP) and why? (LO 5)
The large negative delta G hydrolysis of ATP shows the tendency to transfer its terminal phosphoryl group to water and dissociate to ADP and Pi. (p. 982 of p. 475 of 4th Edition Biochemistry: A Short Course).
This is due to: (Glycolysis case discussion: slide 14 and p. 982-984 of p. 475 of 4th Edition Biochemistry: A Short Course)
1) Electrostatic repulsion
2) Stabilization due to hydration
3) Increase in entropy
4) Resonance stabilization of Pi
Your patient has ingested FDG and has undergone a PET scan. As you examine the scan, you see some areas that have very bright spots. What macromolecule is the scan detecting, and how might a disease cause this buildup of the molecule? (LO 10)
Sugar; buildup may be caused by cancer because the cells use more glucose (Glycolysis case question #10)
What is the structure, electronegativity, reactivity, and stability that make a single carbon compound least useful for fuel and why? (LO 4)
A fully oxidized compound with all C-O bonds is the least useful for fuel. Oxygen has a high electronegativity, so it holds electrons tightly at a low energy level, making these electrons less reactive and more stable (Glycolysis case intro: slide 21 and 25).
Name and explain two general favorable reactions that can be used to couple with unfavorable reactions. (LO 7)
ATP hydrolysis; ATP has a high phosphoryl-transfer potential
Oxidation; a more electronegative atom holding onto the negative charge more tightly (Glycolysis case discussion PowerPoint slides 7 and 13)
What would happen if the thioester intermediate is not able to couple the oxidation and phosphorylation taking place in Step 6 of glycolysis? (LO 8)
The activation energy from the intermediate step would be extremely high. (Glycolysis Case Study Question 4)