What is any characteristic of a material that can be observed or measured without changing the identity of the material? (p.70)
A physical property
What do we call the physical property that is dependent on both temperature and pressure, and occurs in four forms -- solid, liquid, gas, or plasma (p.73)
States of matter
What do we call the ability of a metal to be hammered, pressed, or rolled into thin sheets? (p.76)
Malleability
What is any characteristic that gives a substance the ability to undergo a change that results in a new substance? (p.80)
Chemical property
What is a sign of a chemical change? (p.83)
Forming gas bubbles, heat, light, smoke, change in color, and sound
What is anything that has mass and takes up space? (p.71)
Matter
What do we call the temperature at which a solid becomes a liquid? (p.75)
Melting point
What do we call the ability of a metal to be drawn into wires? (p.76)
Ductility
What is a change in the identity of a substance due to the chemical properties of that substance? (p.81)
Chemical change
No. Chemical changes cannot be reversed.
Physical changes usually can be reversed easily.
What is a change in which the properties of a substance change, but the identity of the substance always remains the same? (p.71)
A physical change
What do we call the temperature at which a substance in liquid state becomes a gas? (p.75)
Boiling point
What do we call the ability of some metals to be attracted by the force of a magnet? (p.76)
Magnetism
What do we call the ability of an object to burn? (p.81)
Flammability
What is the amount of matter in an object? (p.72)
Mass
At what temperature does water in solid form (ice) melt? (p.75)
0° Celsius
What two physical properties can be easily observed by looking at an object? The kids in Pre-K are good at identifying these two properties! (p.71)
Color and shape
What is the form of energy that flows from a warmer object to a cooler object? (p.80)
Heat
Volume
At what temperature does liquid water change into a gas? (p.75)
100° Celsius
True or false?
Matter is made of moving particles. (p.74)
True!
The particles of a solid vibrate in a fixed position. They remain close together and have a definite shape and volume.
The particles of a liquid have enough energy to slide past each other. This allows liquids to take the shape of its container.
The particles of a gas move so quickly they have enough energy to move freely away from other particles. Gas particles will spread to fill out any container.
Different metals react with oxygen to form new substances. Copper will develop a green patina, silver develops a tarnish. What does iron develop? (p.81)
Rust
NOTE: NOT A QUESTION
Physical properties: color, shape, length, mass, volume, density, state, ability to attract a magnet, melting point, boiling point, malleability, ductility
What is the amount of mass that a material has in a given volume? (p.72)
Density
Density = mass ÷ volume
Which state of matter does water not exist in? (p.74)
Water does not exist as plasma.
Water can be a solid (ice), liquid (water), or gas (water vapor).
What is a tool that scientists can use to identify an unknown substance or organism by comparing the physical properties of the unknown substance, selecting between two choices? (p.77-78)
A dichotomous key
What states that the mass of what you end with is always the same as the mass of what you start with? (p.85)
The Law of Conservation of Mass
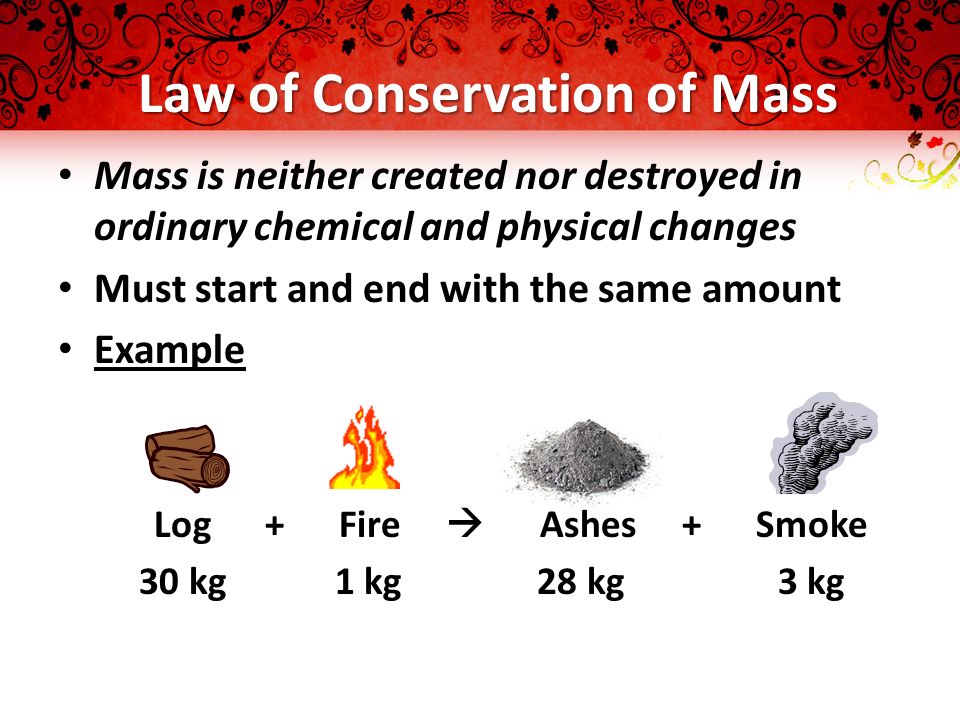
NOTE: NOT A QUESTION
Chemical properties: flammability; reacts with: oxygen, water, vinegar, etc.; reacts in the presence of electricity, light, heat, etc.