Name the following compounds.
MgBr2 and N2F6
What is magnesium bromide and dinitrogen hexafluoride ?
When a strong base like Mg(OH)2 dissociates into its ions, the correct balanced equation is...
Mg(OH)2(aq) --> Mg2+(aq) + 2 OH-(aq)
The scientist known for discovering 6.02 x 1023 entities/mol.
Who is Avogadro? Known for Avogadro's constant, Na = 6.02 x 1023
Name the following acids
HCl(aq) and HClO3(aq)
HCl(aq) Hydrochloric acid and
HClO3(aq) Chloric Acid
Name what each variable stands for and what each is measured in.
n = PV/RT
n --> moles or mol
P --> pressure measured in kPa
V --> volume measured in L
R --> gas constant (8.314)
T --> temperature measured in Kelvin (K)
The correct chemical formula for iron (III) persulfate
What is Fe2(SO5)3 ?
Name the type of reaction and balance.
AlPO4 + BaCl2 --> AlCl3 + Ba3(PO4)2
Double Displacement
2 AlPO4 + 3 BaCl2 --> 2 AlCl3 + Ba3(PO4)2
Mr. G refers to the following sequence as..
Balanced Chemical Equation
Given and Required
Find 'n' or Find moles
Mole Ratio
Find whatever you need
What is the 5 Step Process?
This compound is known to be able to act as an acid AND a base
What is water H2O or H(OH)
In this container, what is the total pressure?
Pred gas = 50 kPa
Pblue gas = 35 kPa
Pyellow gas = 60 kPa
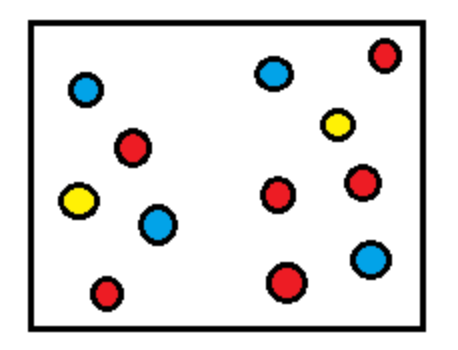
Ptotal = Pred + Pblue + Pyellow
= 50 + 35 + 60
= 145 kPa
Name the following compounds H2CO3(aq) and H2CO2(aq)
What is carbonic acid and carbonous acid ?
You go in your fridge and find 25 strawberries, 4 bananas, and 7 cups of milk. Based on reaction below, how many strawberry banana smoothies can you make? Which ingredient do you run out of first (limiting reagent) ?
5 strawberries + 1 banana + 2 cups of milk --> 1 smoothie
You can make 3 smoothies, and you would run out of milk first.
When a reaction is done in real life, you often don't collect as much product as you would expect. The amount you collect is known as the ____ while the amount you expect to produce is known as the ____.
What is the experimental yield?
which is always less and usually given to you in the question
What is the theoretical yield?
which is found by calculating the mass from the 5 step process
Given the concentration of an acid, describe the steps to find pH.
Step 1 Write the balanced ionization/dissociation reaction.
Step 2 Find the concentration H+ based on the BCE
Step 3 Use pH = -log(H+)
When a reaction is conducted at a temperature of 0 oC or 273 K and a pressure of 101.3 kPa.
When a reaction is conducted at a temperature of 25oC or 298 K and a pressure of 100 kPa
What is STP and SATP?
The chemical formula for silver (II) carbonate * tetra hydrate
AgCO3 * 4 H2O
In the following Total Ionic Equation, which are the spectator ions? What is the net ionic equation?
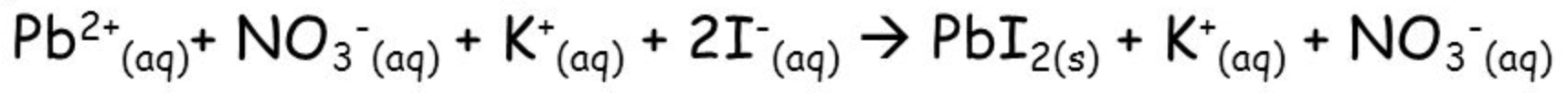
NO3-(aq) and K+(aq) are the spectator ions because they stay as aqueous throughout the reaction.
The net ionic equation is Pb2+(aq) + 2 I-(aq) --> PbI2(s)
Name all 4 ways (4 equations) to solve for n that we learned in this class.
n = # of atoms x Na
n = m/M
n = cv
n = PV/RT
In this reaction, which compound is the acid, which compound is the base?
NH3(aq) + HCl(aq) --> NH4+(aq) + Cl-(aq)
NH3(aq) is the base because it ACCEPTED an H
HCl(aq) is the acid because it DONATED an H
It is your younger sibling's birthday but you are annoyed at them. You hide their balloons in the freezer. Your sibling finds the balloons and starts crying. What happened ? What caused this?
The balloons shrunk in the freezer. This is because when temperature DECREASES, volume also DECREASES.