What are the four main states of matter?
Solids, liquids, gases, and plasmas.
What is the chemical formula for water?
H2O
How many protons does bromine have?
35
The Alkaline Earth Metals are what group number?
Group Two
Which model of the atom was considered to be the "first", and suggested that all elements are simply spheres of different sizes?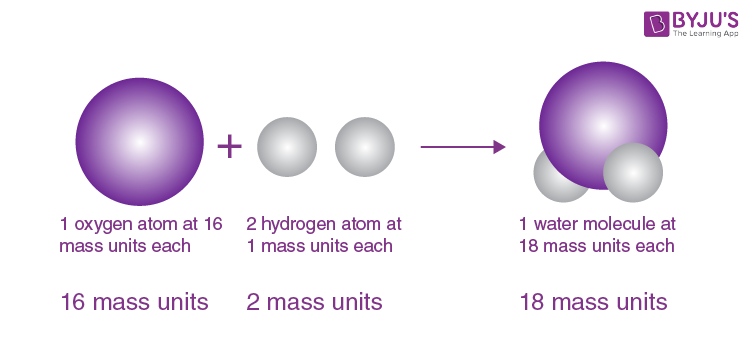
Dalton Model
If an object's density is greater than 1.0g/cm3, what will it do in water?
Sink.
What is the symbol for mercury?
What is Hg
How many electrons does chromium have?
24
How many elements are considered Noble Gases?
Six (helium, neon, argon, krypton, xenon, and radon).
The Thomson model of the atom introduced which subatomic particle?
The electron.
What is the correct term for changing from a gas to a solid?
Deposition.
What element uses the symbol: Mn
Manganese
What two subatomic particles are found in the nucleus?
Protons and neutrons.
Is phosphorus a metal or non-metal?
Non-metal
What element is represented by the following Bohr Model: 
Chlorine (Cl)
What type of chemical reaction occurs when an acid and base are mixed?
Neutralization (or double replacement).
What is the symbol for antimony?
Sb
What is the mass number for niobium?
93 (atomic mass = 92.9)
Groups 3 through 12 on the Periodic Table are referred to as:
The transition metals
Who introduced the neutron to the atom? Hint: You can also use his nickname (for half points).
James Chadwick a.k.a. Jimmy Neutron
What type of flammable gas is released when an alkali metal reacts with water?
Hydrogen
What is the latin name for lead?
Plumbum
When sodium becomes an ion, how many electrons will it have?
10
How many electron shells does tungsten have?
Six
What does the following model represent? 
The ion form of oxygen.