This is the type of acid that a Bronsted Base forms by accepting a proton
What is a conjugate acid?
this is the central atom in CH2F
What is Carbon?
A bond or molecule whose ends have opposite charges
What is a Dipole?
These numbers are always considered significant figures
what is a nonzero digit?
This is what Lewis acids always accept
what is an electron
The ability of an atom to hold and attract electrons
What is Electronegativity?
The weakest intermolecular force
What is London Dispersion Force?
a 0 between two significant digits is considered this
What is significant?
What is an acid?
this theory is what determines molecular geometry
What is VSEPR or Valence Shell Electron Pair Repulsion theory?
This type of molecular vibration cannot absorb infrared radiation
What is symmetrical stretching?
the number 1200 has this many significant figures.
what is 4?
When you use a solution of a known concentration to find the concentration of an Unknown solution
What is Titration?
The molecular geometry of a molecule with two lone pairs and one double bond off of the central molecule.
What is Trigonal Planar?
These are the three types of molecular vibration
What is Asymmetric stretching, Symmetric stretching and bending?
the zeroes at the end of the number 1.2000 are considered this.
What is significant?
This is what pH stands for.
What is potential of Hydrogen
This is the proper name for this molecular geometry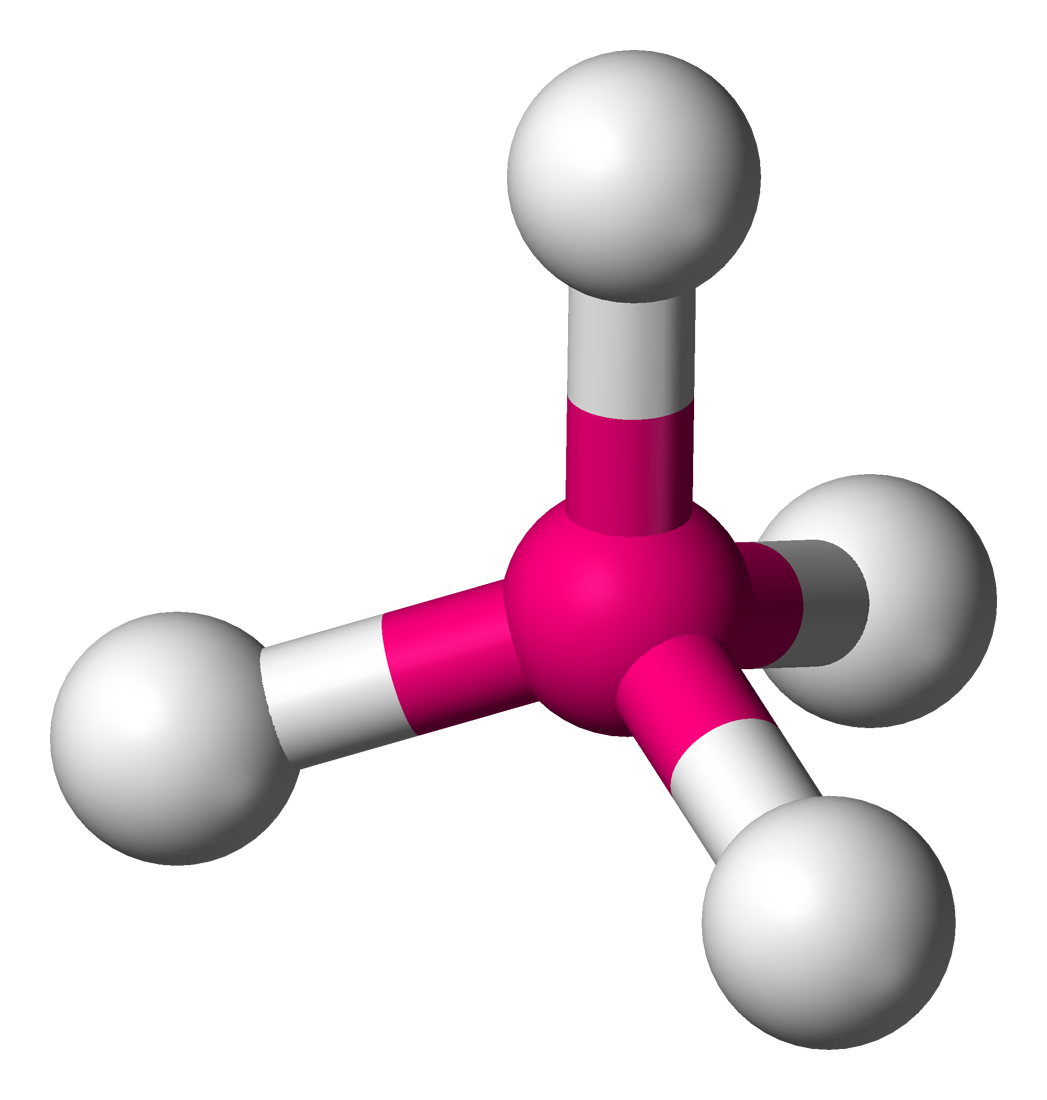
What is Tetrahedral?
In addition to the radiation energy being equal to the energy required to vibrate a molecule, this must also be true to absorb infrared radiation.
the vibration leads to the dipole moment of the molecule changing
the number 101.0250020 has this many significant figures
What is 10?