The 4 different symbols that represent matter in a reaction?
What is s, g, l, aq.
This type of bond is has the possibility of being polar
What is covalent bond?
The geometric name for a compound with 4 bonding pairs and no lone pairs.
What is Tetrahedral?
The name of a chemical that is bound to water.
What is hydrate?
The coefficient in a balanced reactions represents this.
What is the number of moles present of a chemical?
The skeleton formula for a synthesis reaction.
What is A + B --> AB
This type of bond is not capable of being polar. (2 possible answers)
What is ionic bond?
What is metallic bond?
The geometric name for a compound with two bonding pairs and two sets of lone pairs.
What is bent?
What is tetrahydrate?
The balanced equation for the following reaction:
__CH4 + __O2 --> __CO2 + __ H2O
What is:
1CH4 + 2O2 --> 1CO2 + 2H2O
The type of reaction that produces CO2 and H2O.
What is combustion reaction?
The polarity of O2.
What is non-polar?
 The name of this geometric shape.
The name of this geometric shape.
What is trigonal planar?
What is Sodium Chloride Hexahydrate
The balanced equation for the following reaction:
__ H2O + __CO2 --> __C6H12O6 + __O2
What is:
6H2O + 6CO2 --> 1C6H12O6 + 6O2
The type of reaction that is AB + CD --> AD + CB.
What is Double-Replacement?
The polarity of HCN
What is polar?
The name of this geometric shape.
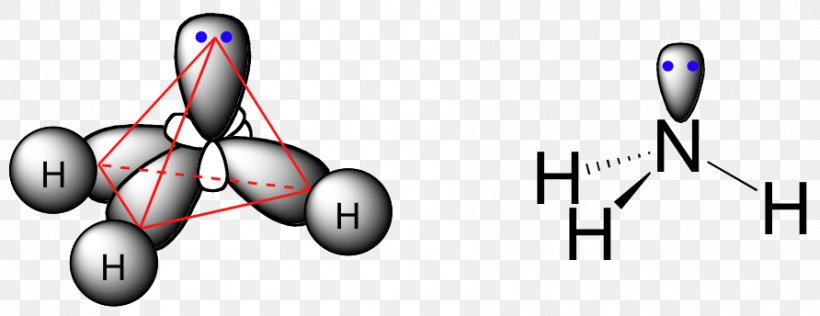
What is trigonal pyramidal?
The term used for a salt that has lost the water connected to it.
What is anhydrous?
Given the following reaction:
C3H8 + 5O2 --> 3CO2 + 4H2O
This is the amount, in grams, of CO2 that are formed from 11.0 g O2.
The products for the following Double-Replacement reaction: NaCl + AgNO3 -->
What is NaNO3 + AgCl?
This description of geometry tells us that a molecule is going to be non-polar.
What is symmetrical?
The geometric shape of CCl4.
What is tetrahedral?
The empirical formula of a molecule containing 49.8 g sodium, 113.6 g chromium, and 121.4 g oxygen.
What is Na2Cr2O7
DAILY DOUBLE:
Take the reaction: NH3 + O2 --> NO + H2O. In an experiment, 3.25 g of NH3 are allowed to react with 3.50 g of O2.
This is the mass of NO that is produced.
What is 2.63 g NO?