The following reaction is an example of what type:
2C5H10 + 10O2 --> 5CO2 + 10H2O
Combustion
What is the molar mass of aluminum nitrate - Al(NO3)3?
213.01 g/mol
A solution with a pH of 5.0 is ______________
Acidic
In every balanced chemical equation, each side of the equation has the same number and type of:
Atoms
How many molecules does one mole of carbon contain?
6.02x1023
Is an example of this type of reaction.
4Fe + 3O2 --> 2Fe2O3
Synthesis/Combination
N2 + 3H2 → 2NH3
The amount of moles of hydrogen needed to react with 2 moles of nitrogen.
What is 6 moles of H2?
Ammonia has a pH of 13. Ammonia is a(n) __________.
Base
What type of radiation is most penetrating?
Gamma
What type of reaction is the following
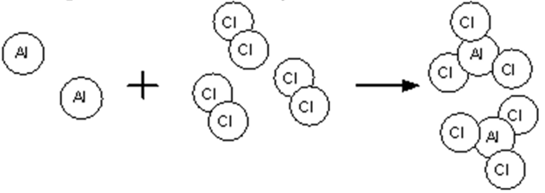
Synthesis/Combination
Is an example of this type of reaction.
AgNO3 + NaCl ---> AgCl + NaNO3
double replacement
Use the following equation:
2Al + 3Cl2 → 2AlCl3
If 140 grams of aluminum chloride react, how many moles of aluminum will be produced?
1 mole AlCl3 = 133.34g AlCl3
1.05 mol of Al
Substances can be acidic, basic or neutral in pH. Arrhenius bases contain _____. (What type of ion?)
OH- ions
The symbol for a substance dissolved in water is
(aq) - aqueous
Which is the product of the following reaction?
Mg + N2 -> ?
Mg3N2
Balance the following equation
___ Sb + __ O2 --> __ Sb4O6
4,3,1
What are the three conversion factors that are equal to one mole? (Liters, Grams, and Atoms)
22.4L
Molar Mass
6.02x1023
The molarity of a solution refers to the number of moles of solute per...
The following generic equation is an example of....
AB -> A + B
Decomposition
New substances formed in a chemical reaction are called
products
Which type of reaction can be recognized by the following general pattern?
A +BX --> AX + B
Single Replacement
Consider the balanced equation below. 4.7 moles of silver nitrate will produce how many moles of silver chloride?
3 𝐴𝑔𝑁𝑂3+𝐴𝑙𝐶𝑙3→3 𝐴𝑔𝐶𝑙+𝐴𝑙(𝑁𝑂3)3
4.7 moles of Silver Chloride
If you dissolve 20.0g of sodium hydroxide (NaOH) in 250mL if water what is the molarity of the solution?
2.00M
Balance the following equation:
___CH4 + ___S8 -> ___ CS2 + ___H2S
2,1,2,4
What are the correct coefficients when the following equation is balanced?
____K + ____Br2 ->____KBr
2,1,2