Compare the average kinetic energy of the molecules of the sample during interval BC to the average kinetic energy of the molecules of the sample during interval DE.
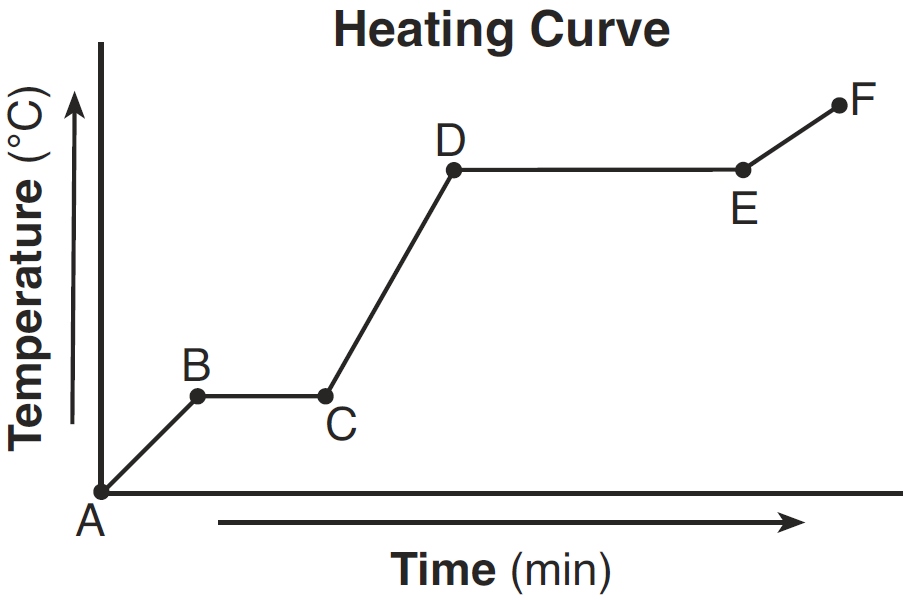
- The average kinetic energy of the molecules during interval BC is less than the average kinetic energy of the molecules during interval DE.
— During interval DE, the average kinetic energy is higher.
State what happens to the average kinetic energy of the molecules in the sample during the first 3 minutes.
— The average kinetic energy decreases.
— The average KE goes down.
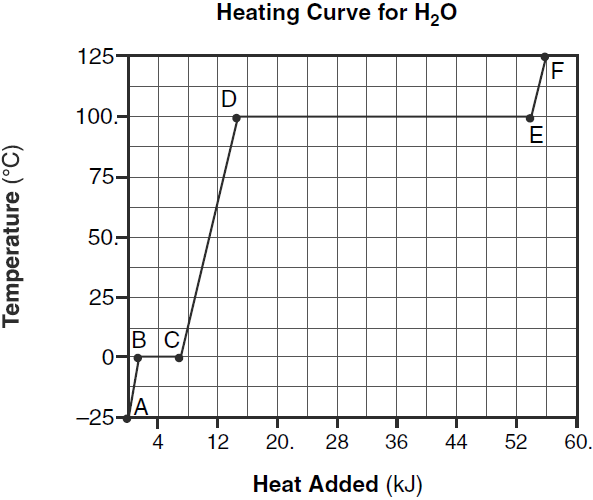
Show the numerical setup that can be used to calculate the heat energy required to completely melt 125. grams of H2O(s) at 0°C?
Q=mHf= (125)(334)
Which statement defines the temperature of a sample of matter?
A) Temperature is a measure of the total electromagnetic energy of the particles.
B) Temperature is a measure of the total thermal energy of the particles.
C) Temperature is a measure of the average potential energy of the particles.
D) Temperature is a measure of the average kinetic energy of the particles.
D) Temperature is a measure of the average kinetic energy of the particles.
During which two processes does a substance release energy?
A) freezing and condensation
B) freezing and melting
C) evaporation and condensation
D) evaporation and melting
A) freezing and condensation
State what happens to the potential energy of a sample of NO(l) at 121 K as it changes to NO(g) at constant temperature and standard pressure.
The potential energy increases
Describe what happens to both the potential energy and the average kinetic energy of the molecules in the H2O sample during interval AB.
–The potential energy remains the same, but the average kinetic energy of the H2O molecules increases.
–There is no change in potential energy. There is an increase in the average kinetic energy.
Which segment of the graph represents the gas phase, only?
– AB
– from time 0 to 2 minutes
Show a numerical setup for calculating the quantity of heat in joules required to completely vaporize 102.3 grams of H2O(l) at 100.°C and 1.0 atm.
— q = (102.3 g)(2260 J/g)
— 2260 x 102.3
The particles in which sample have the lowest average kinetic energy?
A) 50. g of sulfur at 273 K
B) 40. g of aluminum at 298 K
C) 30. g of sulfur at 303 K
D) 20. g of aluminum at 323 K
A) 50. g of sulfur at 273 K
Heat flows from an object at a temperature of 20.°C to an object at a temperature of
A) 15°C
B) 25°C
C) 35°C
D) 45°C
A) 15°C
At 1 atm and 190 K, compare the amount of thermal energy in a 1.0-kilogram block of dry ice to the amount of thermal energy in a 2.0-kilogram block of dry ice.
– The block of dry ice with less mass contains less thermal energy.
– There is more thermal energy in the 2.0-kg block.
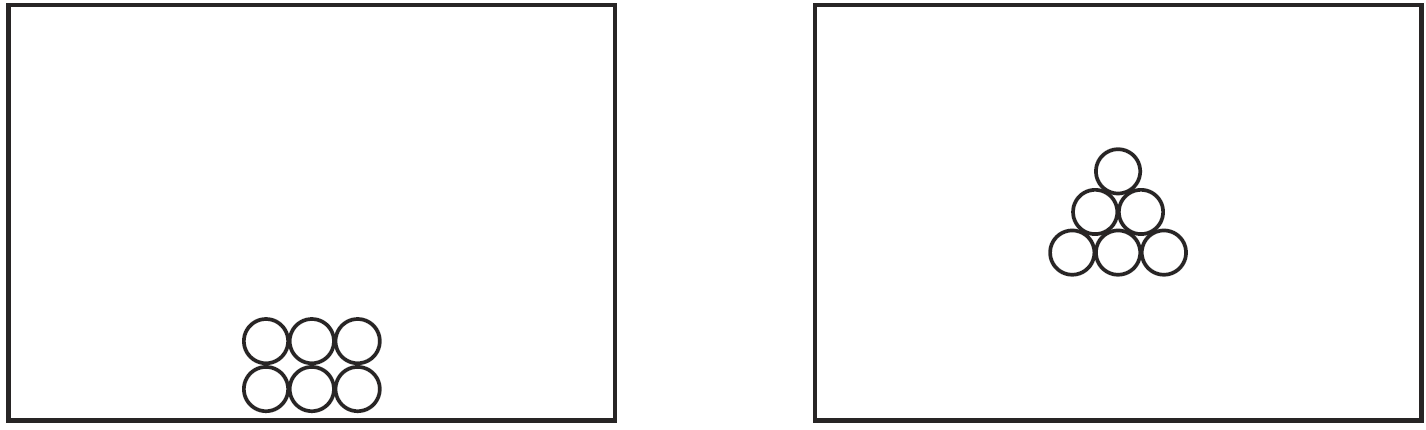
Draw a particle diagram to represent the sample during interval AB. Your response must include at least six molecules

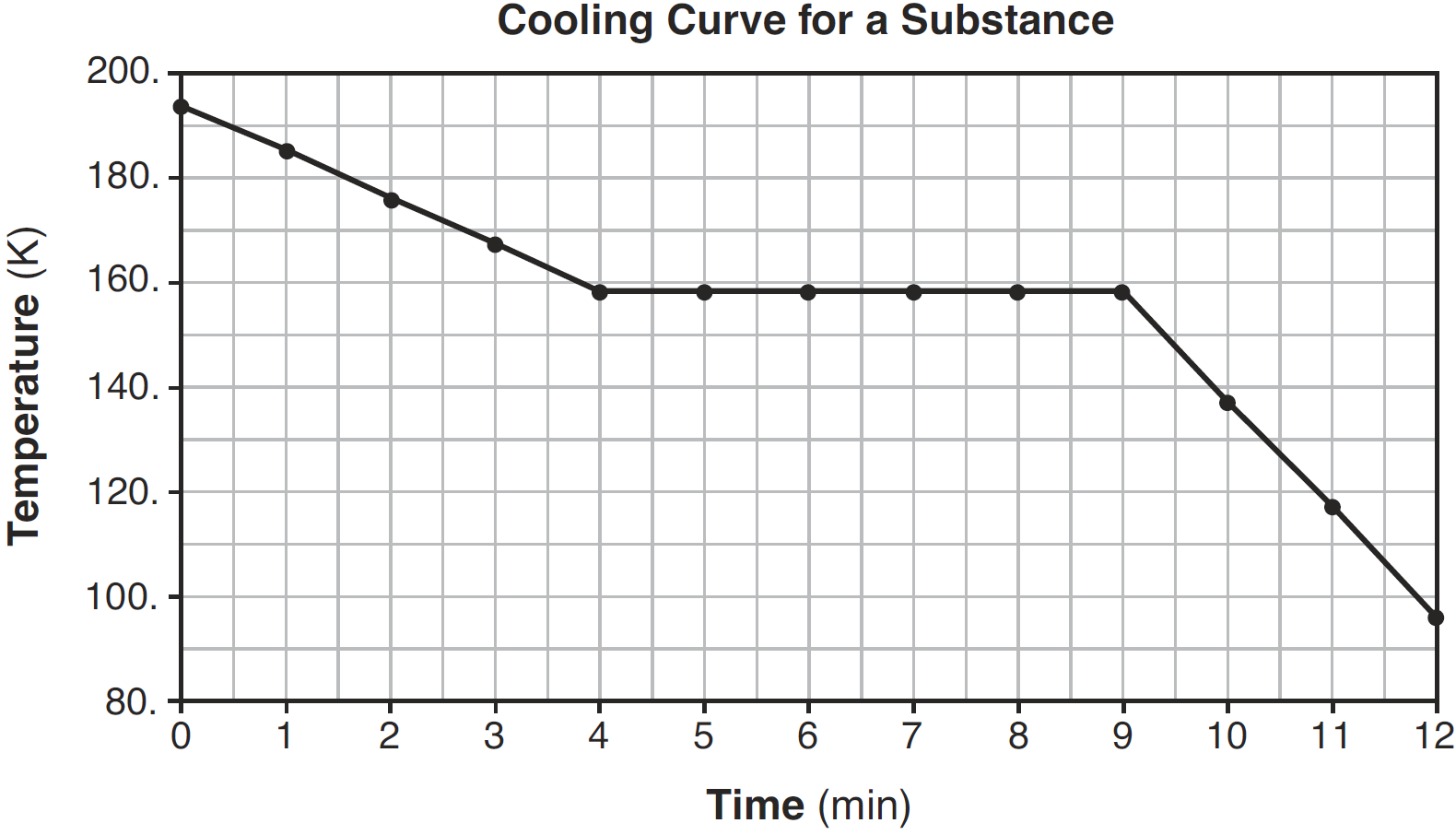
What is the melting point of this substance?
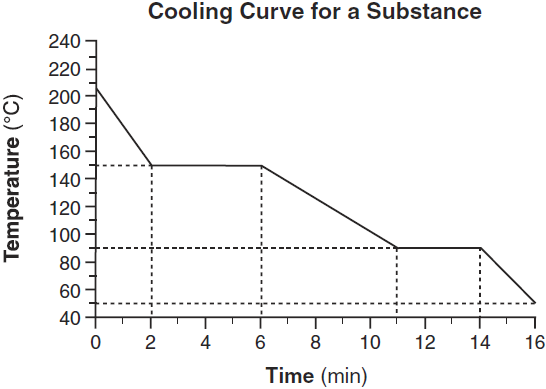
90oC
What is the amount of heat, in joules, required to increase the temperature of a 49.5-gram sample of water from 22°C to 66°C?
9104J
Q= (49.5)(4.18)(44)
Which temperature represents the highest average kinetic energy of the particles in a sample of matter?
A) 298 K
B) 267 K
C) 27°C
D) 12°C
C) 27°C (300K)
Which form of energy is transferred when an ice cube at 0°C is placed in a beaker of water at 50°C?
A) chemical
B) electrical
C) nuclear
D) thermal
D) thermal
Which process increases the potential energy of the particles of a sample?
A) condensation
B) deposition
C) solidification
D) vaporization
D) vaporization
What is happening to the average kinetic energy of the particles during segment BC?
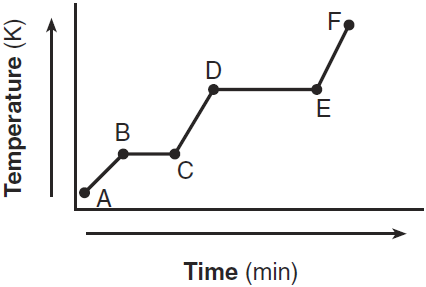
It does not change.
During which time interval does the substance exist as both a liquid and a solid?
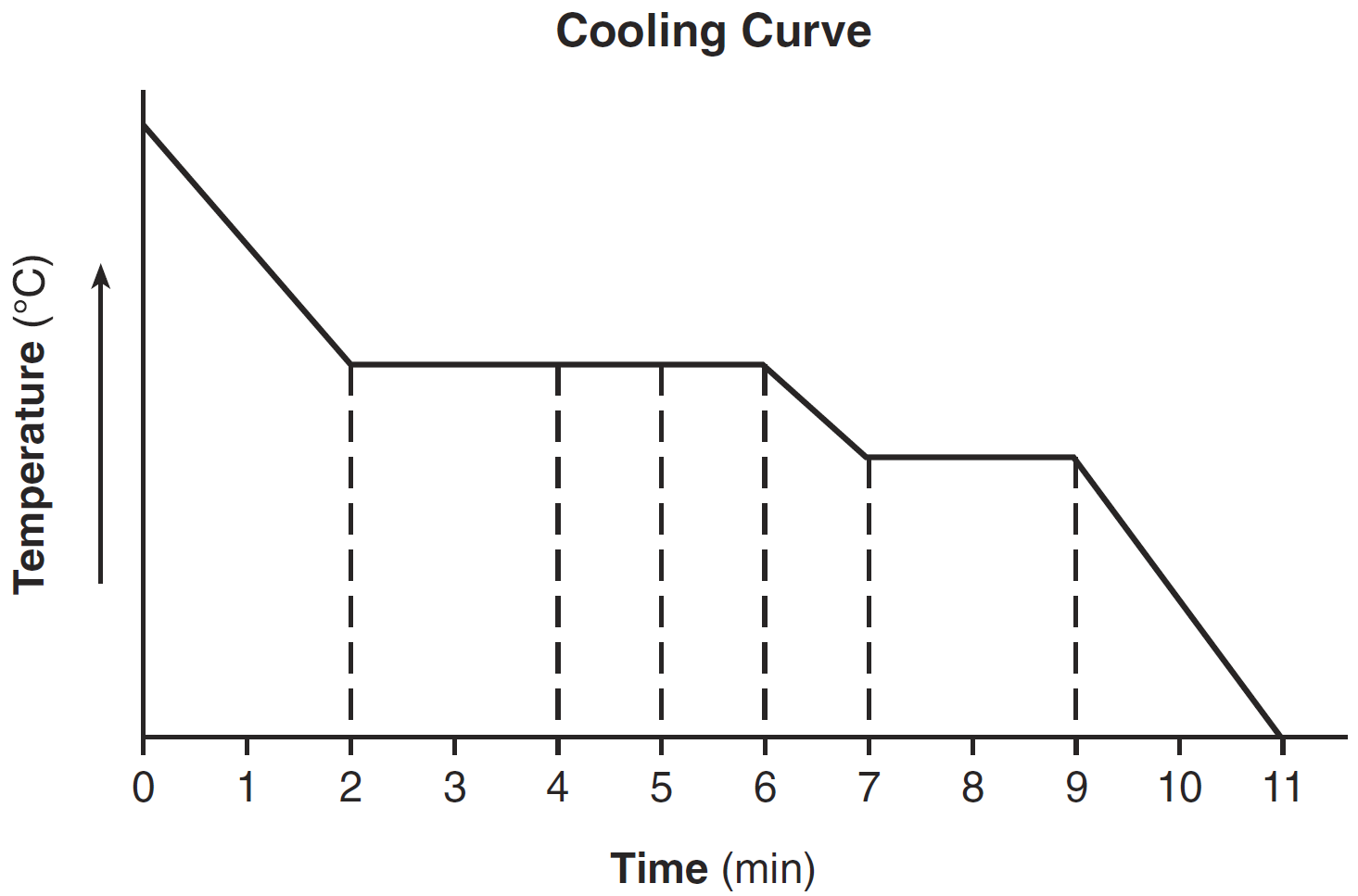
7-9 minutes
Determine the total amount of heat, in joules, required to completely vaporize a 50.0-gram sample of H2O(l) at its boiling point at standard pressure.
113,000J
Q=(50)(2260)
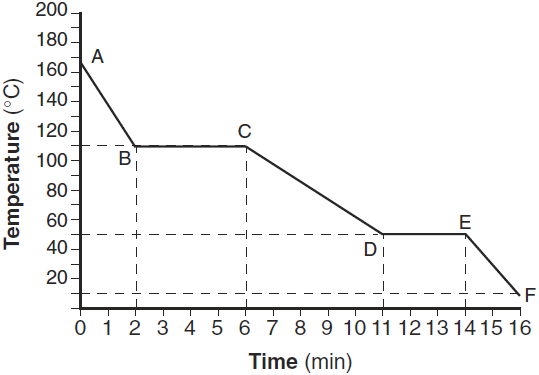
Convert 120. K to Celsius
-153oC
In a laboratory where the air temperature is 22°C, a steel cylinder at 100.°C is submerged in a sample of water at 40.°C. In this system, heat flows from
A) both the air and the water to the cylinder
B) both the cylinder and the air to the water
C) the air to the water and from the water to the cylinder
D) the cylinder to the water and from the water to the air
D) the cylinder to the water and from the water to the air
Convert the melting point of mercury to degrees Celsius.
–39°C
Identify the process that takes place during line segment DE of the heating curve
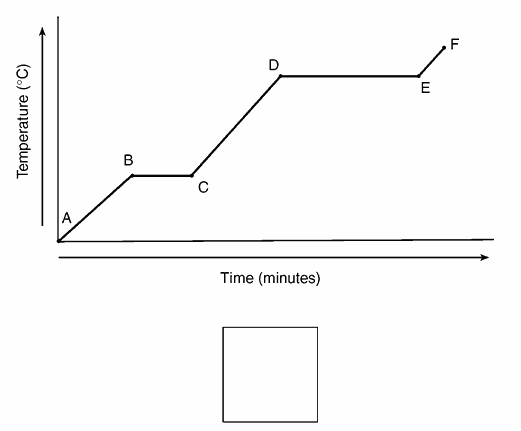
–boiling –vaporization –liquid – vapor equilibrium
Which segments of the cooling curve represent the fixed points on a thermometer?
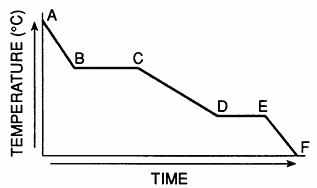
BC and DE
Compare the amount of heat required to vaporize a 200.-gram sample of H2O(l) at its boiling point to the amount of heat required to melt a 200.-gram sample of H2O(s) at its melting point.
It takes more heat to vaporize the same amount of H2O(l)
A liquid's freezing point is –38ºC and its boiling point is 357ºC. What is the number of Kelvin between the boiling point and the freezing point of the liquid?
395K
Compare the thermal energy of a 10.-gram sample of water at 25°C to the thermal energy of a 1000.-gram sample of water at 25°C
- The thermal energy is greater for the 1000 g sample of water.
-The smaller sample has less thermal energy.
Describe what happens to the potential energy and the average kinetic energy of the molecules in the sample during interval DE.
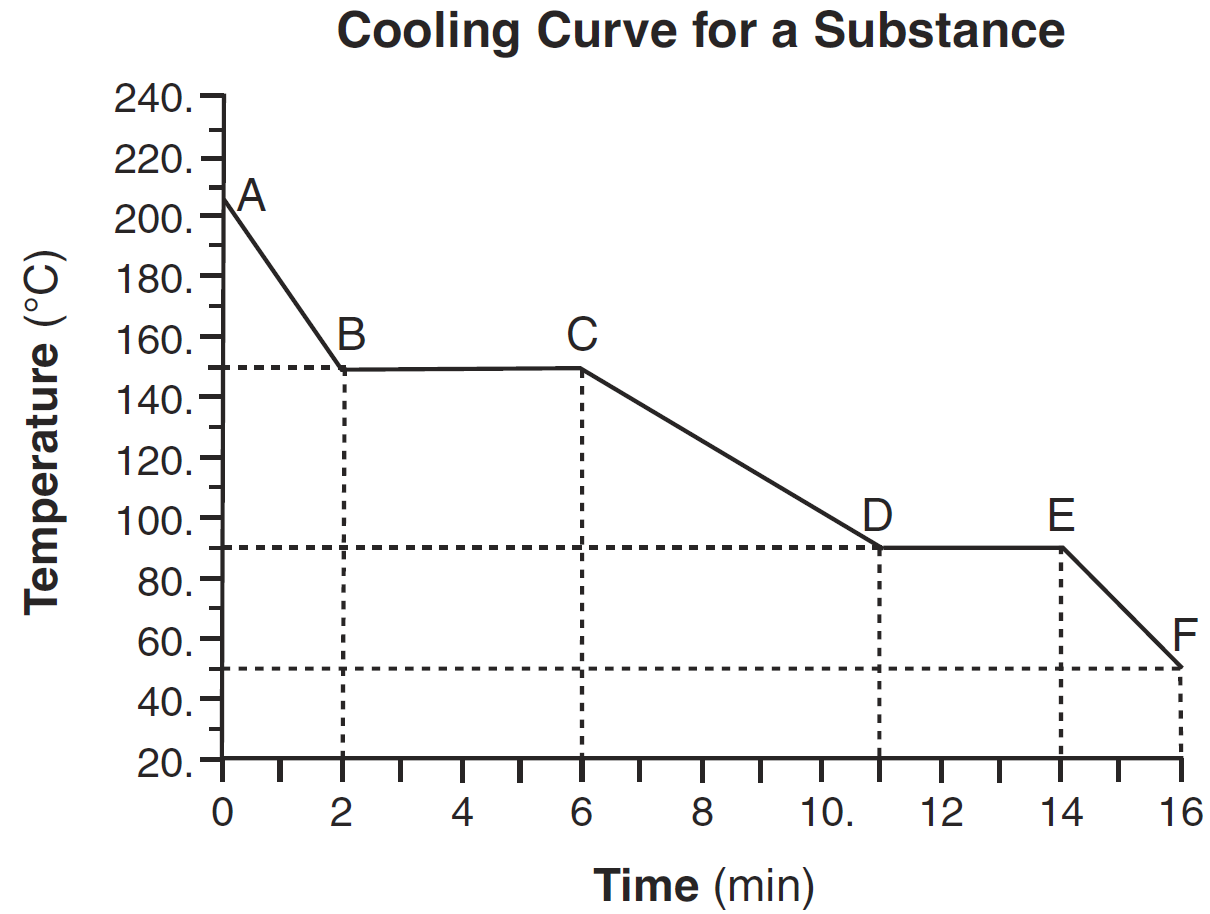
— Potential energy: decreases — Average kinetic energy: no change
— Potential energy: There is a decrease.— Average kinetic energy: It remains the same.