Which element has the greatest attraction to electrons when bonded to Na?
1. N
2. O
3. S
4. Al
What is 2. O
The type(s) of elements that must be included in a covalent compound (metals, nonmetals, or both)
Which statement best describes the reaction H + H --> H2 + energy?
1. A bond is broken, which absorbs energy
2. A bond is formed, which absorbs energy
3. A bond is broken, which releases energy
4. A bond is formed, which releases energy
What is 4. A bond is formed, which releases energy
As liquid water boils at standard pressure, the temperature remains at 100 degrees Celsius until it has completely evaporates. During this constant temperature, the potential energy _________.
What is Increases?
What is the name of the following compound:
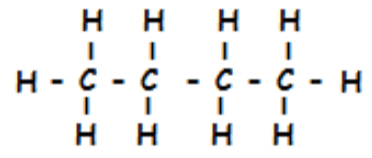
1. propane
2. butane
3. propene
4. butene
What is 2. butane
The number of valence electrons that the halogens contain.
What is 7
Which of the following substances is the best conductor of electricity when dissolved in water?
1. K2SO4
2. CCl4
3. C6H12O6
4. NO2
What is 1. K2SO4
What is the molar ratio of CO2 to H2O in the following balanced chemical equation:
CH4 + 2O2 --> CO2 + 2H2O
What is 1:2 ?
Which phase change represents an exothermic process: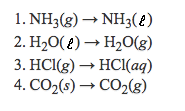
What is 1. NH3(g) --> NH3(l)
The temperature value at which the substance on this graph condenses.
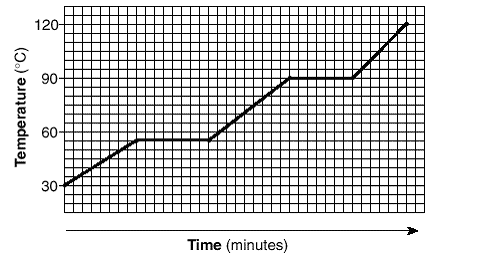
What is 90 oC?
Alkali Earth metals will lose this many electrons to form an ion.
What is 2
In the molecule CH3Cl, which element represents the partially negative end of the molecule?
What is Cl
Which chemical reaction results in the formation of one large compound from two or more smaller reactants?
1. single replacement
2. double replacement
3. synthesis
4. decomposition
What is 3. synthesis?
The temperature range at which this substance is a liquid: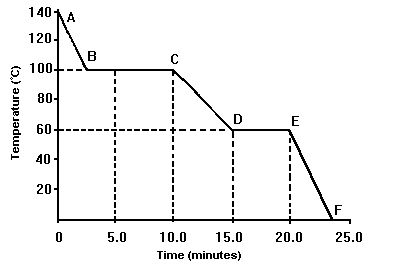
What is 60 oC to 100 oC?
The freezing point of chlorine in Celsius.
What is -101 oC?
State the trend (increase/decrease) in first ionization energy as elements in Group 1 are considered in order of increasing atomic number.
What is decreasing?
The name of the compound with the formula Cu(NO3)2 is ...
1. copper nitride
2. copper (II) nitride
3. copper nitrate
4. copper (II) nitrate
What is 4. copper (II) nitrate
Which equation shows conservation of mass?
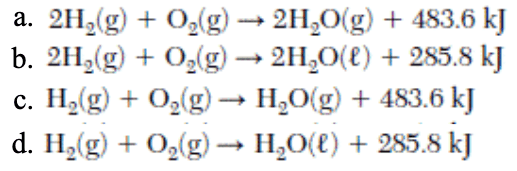
What is a. or b.
How much heat energy must be absorbed to completely melt 45.0 grams of H2O(s) at 0°C?
What is 15,030 J ?
What does HCl do in solution that makes it be considered an acid?
What is yields an H+ in solution?
What is donates a proton in solution?
Which properties are characteristic of Group 2 elements at STP?
1. good electrical conductivity and electronegativities less than 1.7
2. good electrical conductivity and electronegativities greater than 1.7
3. poor electrical conductivity and electronegativities less than 1.7
4. poor electrical conductivity and electronegativities greater than 1.7
What is 1. good electrical conductivity and electronegativities less than 1.7
What is the percent composition of silver in AgNO3?
What is 63.5% (range of 62%-65%) ?
State evidence from the following equation that this reaction is exothermic:
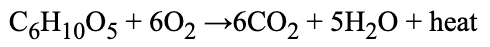
What is heat is released?
What is heat is a product?
What is heat is on the right side of the equation?
This line segment represents the solid phase only.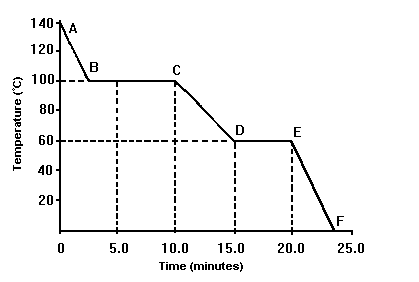
What is EF?
Which of the following can undergo an addition reaction?
1. C3H8
2. C4H8
3. C5H12
4. CH4
What is 2. C4H8