What term refers to the randomness of a substance or system?
Which contains more energy: 50g of boiling water at 100*C or 50g of steam at 100*C?
50g of steam at 100*C

According to the 2nd law of thermodynamics, the entropy of the universe is ______________.
In a laboratory experiment, 30. J of heat was added to a gas piston assembly as the piston did 75 J of work on its surroundings. Calculate AE for this system.
Q + W
30. J + (-75 J) =
= -45 J
Heat flows from a _______ temperature to a ________ temperature.
higher to lower

What is the internal energy of a system plus the product of its pressure and volume?

enthalpy
Internal energy is a _______ function, so it equals the DIFFERENCE of the ________ value and the ________ value.
state
final
initial

The enthalpy of formation for any ELEMENT in its standard state is: ____________.

A 15.0 g piece of graphite is heated to 100.0°C and placed in a calorimeter. The graphite releases 815.1 J of heat to reach a final temperature of 23.9°C. What is the specific heat of graphite?
Q = mcAT ---> c=Q/mAT
-815.1 J/ (15.0g)(-76.1*C) =
= .714 J/g x *C
Lava turning to a solid, would this most likely increase or decrease in entropy

decrease (solids usually have the least disorder)
How is energy commonly defined?

the ability to do work
When baking soda (sodium bicarbonate, NaHCO3) reacts with acetic acid (HC2H302) from vinegar to form carbon dioxide and water, the reaction mixture warms slightly. Which one is less, the enthalpy of the products or of the reactants.
the enthalpy of the products
At constant pressure, enthalpy change equals ___________.
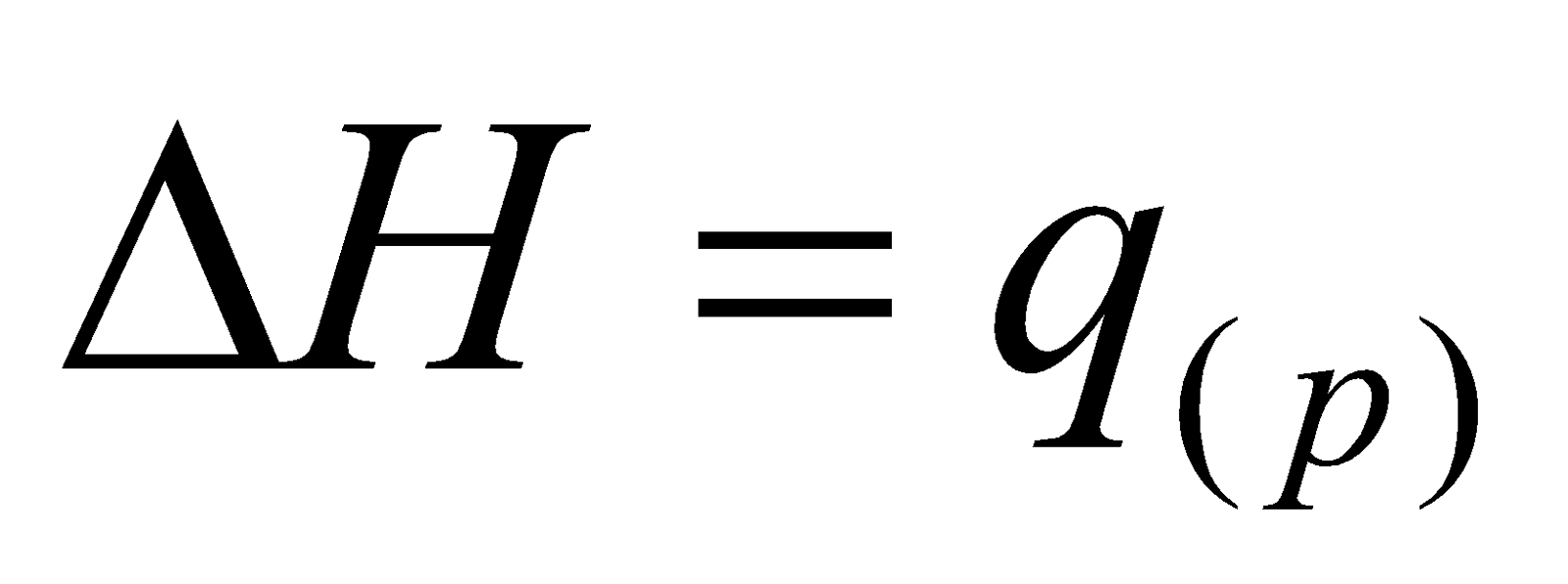
heat
In the presence of some metals, ethylene (C2H4) and hydrogen gas react to form ethane (C2H6) according to the following thermochemical equation:
C2H4(g) + H2(g) --->C2H6(g) AH* =-136,98 kJ
If the molar mass of ethylene is 28.05 g/mol, how much heat is liberated when 100.0 g of ethylene reacts?
-488.3 kJ (released)
Standard state:
_____ atm
_____ K
1 atm
298 K
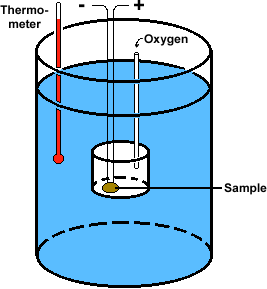
What is a calorimeter used for? (by definition)
measuring heat flow during physical process or chemical reactions
Is heat absorbed or released when water at 0*C changes to ice at 0*C?

released
the heat per unit mass required to vaporize a substance at its normal boiling point is: ________ ____ _____________.
heat of vaporization

How much heat is required to convert 20.0 g of ice at -50.0°C to liquid water at 0.0°C?
The specific heat of ice is 2.06 J/(g•°C) and the heat of fusion of water is 334 J/g.
8740 J
Chemical and physical changes that release heat are known as _____________ processes.
EXOthermic
The _______ law of thermodynamics states that the change in a system's internal energy is the SUM of the ___________ added to the system and the _________ done on the system.
first
heat
work

In an experiment to determine the energy provided by a food product, a sample of the food is burned in a pressurized container at constant volume; 100 J of heat are released. Because volume is constant, the system does no work on its surroundings. Is the change in internal energy positive, zero, or negative?
negative
the amount of heat required to cause a unit rise in temperature of a unit mass of a substance is _________ ____________.

specific heat
Under certain conditions, nitrogen gas and oxygen gas react to form the toxic brown gas nitrogen dioxide: N2 (g) + 2 02(g) → > 2 NO2 (g). For this reaction, AH° = 66.2 kJ and AS° = -121.81 J/K; calculate AG° at standard conditions (298 K and 1 atm) and determine it the reaction is spontaneous, nonspontaneous, or in equilibrium at these conditions.
102.5 kJ; nonspontaneous
Chemical and physical changes that absorb heat are known as _____________ processes.
ENDOthermic
