Heat transfer through direct contact
Conduction
the average kinetic energy (speed) of the particles within a substance
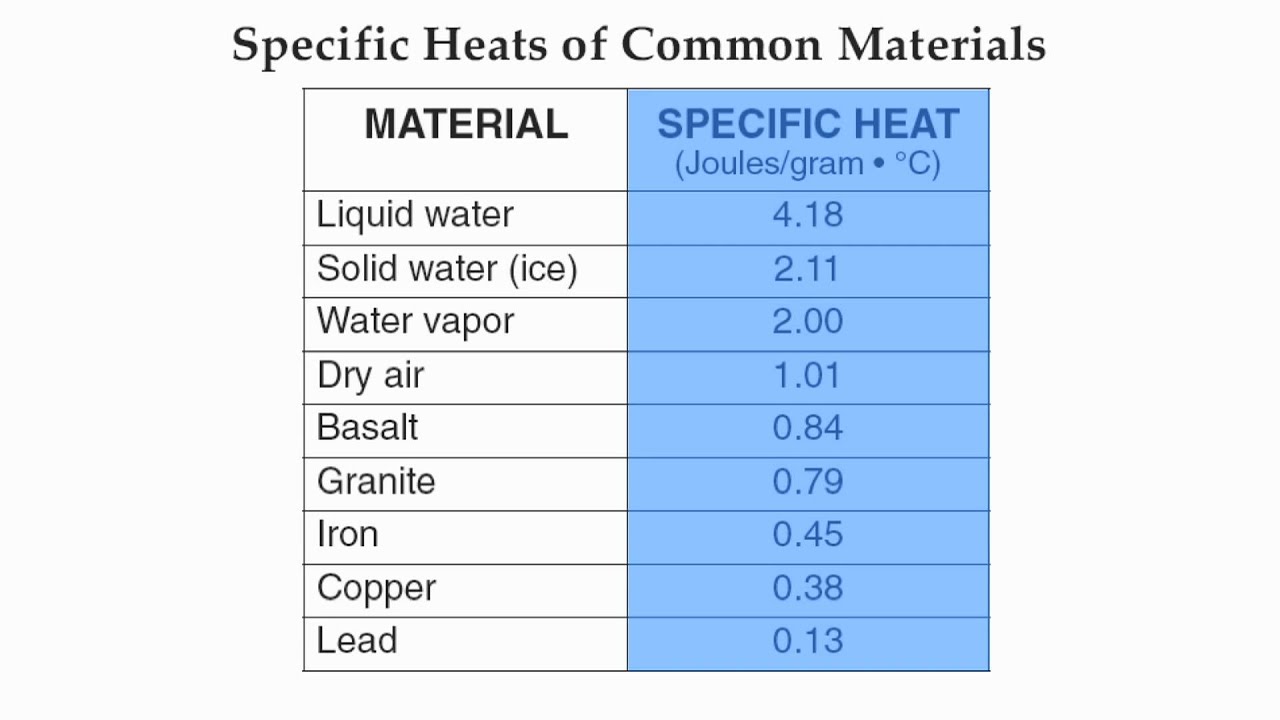
specific heat
Independent variable is the one that I....
Which of the following has the greatest thermal energy?
Large iceberg, hot cup of coffee, small warm puddle of water
Large iceberg
Heat transfer through bulk movement of matter
Convection
Thermal Energy
the total potential and kinetic energy in an object
The unit for energy is
calories (or Joules)
controlled variables
A system that does not allow energy to be lost the environment is called a
closed system
Heat transfer through outer space
Radiation
Heat
How much energy would it take to increase 50 g of water by 20 degrees Celsius?
1000 calories
Identify the dependent variable in this experiment:
Olivia wants to test to see how amount of sunlight affects the growth of her sunflowers. She places one in the shade, one in partial sun, and one in full sunlight, then measures the height of the flowers after 2 weeks.
Is this an open or closed system?
Conduction
The amount of thermal energy required to raise the temperature of 1g of a substance 1 degree Celsius
How much energy would be lost by 10 g of iron (specific heat = .11 cal/g° C) that cooled from 120° C to 60° C ?
66 calories
A student wants to see if studying with different types of music will increase her math test scores. On day 1 she plays classical music for 20 minutes, day 2 she played rap music, and day 3 she played country music.
Two controlled variables: 20 minutes, same test
High, slow, slow
This type of heat transfer works occurs in liquids and gases
Convection
Energy source and energy sink
Source - warmer object, sink - colder object
How much energy would have to be added to raise the temperature of 8 g of gold (specific heat = 0.031 cal/g°C) from 10 degrees Celsius to 90 degrees Celsius.
19.84 calories
Identify the independent and dependent variables:
How does the temperature of water affect how fast sugar dissolves?
Independent: temperature of water
Dependent: speed of sugar dissolving
Which of the following would transfer heat the quickest?