This is why your feet feel warm on a rug and cool on tile.
What is because the tile is a conductor and the rug is an insulator?
This is the definition of temperature.
What is a measure of molecular motion?
When something floats, it has this kind of buoyancy.
What is positive?
As a liquid evaporates off a towel, these molecules leave the towel and these molecules stay on the towel.
What is high KE molecules leave and low KE molecules stay?
The apparent charge build up on either side of a water molecule.
What is water polarity?
A convection current is best described as the _______ (rising/falling) of a heated fluid and the ________ (rising/falling) of a cooler fluid due to density differences.
What is "rising" and "falling"?
This many calories are expended when heating 80 mL of water by 60oC. Note: water's specific heat is 1 cal/goC.
What is 4800 calories?
If you increase the mass of a substance, without changing the volume, the density will change in this way.
What is increase?
When a gas turns into a liquid, the temperature changes in this way, the density changes in this way, and the volume changes in this way.
When a molecule is attracted to a different molecule.
What is adhesion?
The radiation from the sun heats the surface of the Earth. It is hotter at the equator because the rays of the sun strike the earth at a _________ (smaller/larger) angle from the perpendicular, resulting in a ___________ (smaller/larger) area of heat absorption.
What is "smaller" and "smaller"?
This is the unit of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius.
What is a calorie?
A piece of copper has a density of 0.09 g/cm3 and a volume of 34 cm3. What is the mass of the copper?
What is 3.06 grams?
The heat being added to boiling water at 100oC is doing this.
What is evaporating the water?
When a molecule is attracted to another molecule of the same type, and the specific term used for when a water molecule is attracted to another water molecule.
What is cohesion and surface tension?
During the melting ice lab, the heat that melted the ice came from this location.
What is the aluminum block?
A calorimeter helps scientists measure this quantity.
What is heat?
Two objects with the same density are placed in two beakers filled with different liquids with different densities. The liquid in this beaker is more dense.
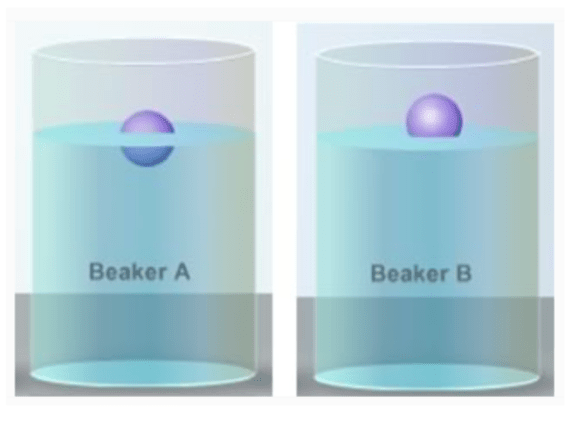
What is B?
Bubbles in boiling water are made of this.
What is water vapor?
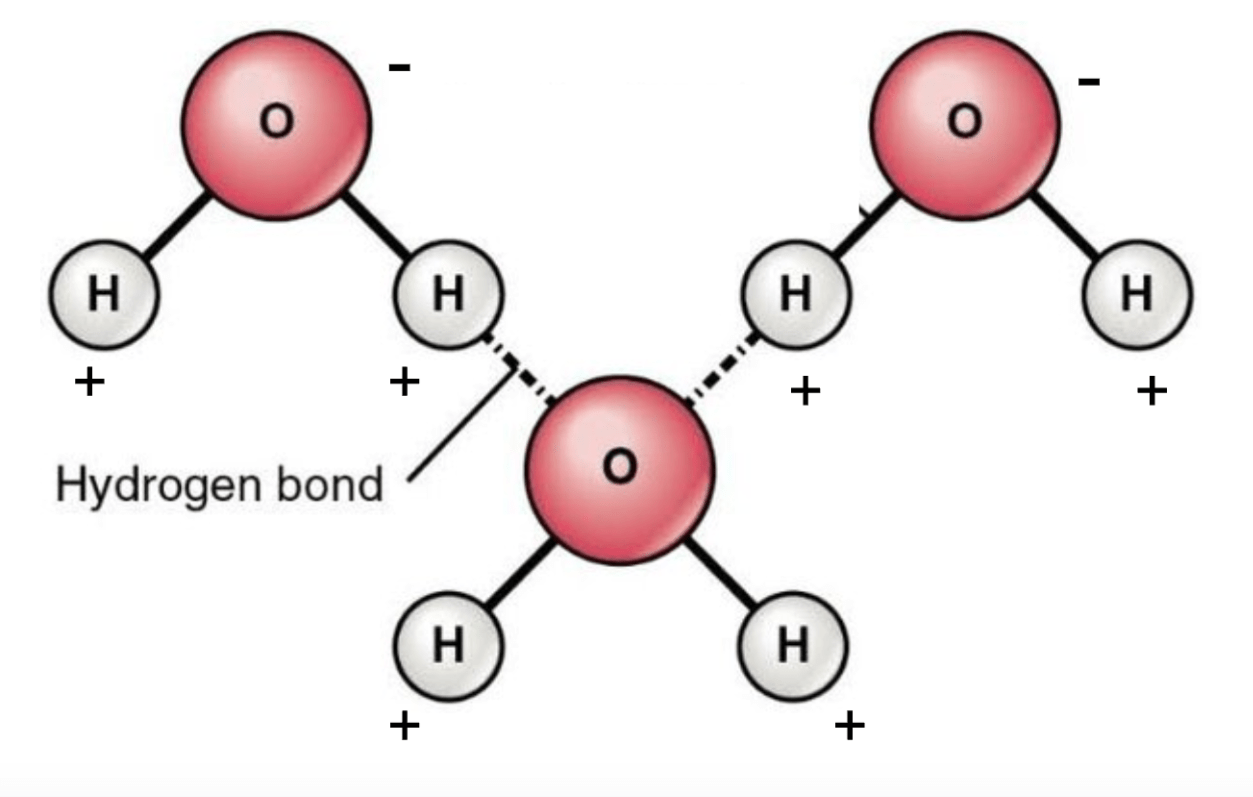
Draw 3 H2O molecules connected by hydrogen bonds. Label the oxygen, hydrogen, positive and negative sides of the molecule, and the bonds.
What is
In the heat transfer lab, this was transferred from the hot calorimeter cup into the cool calorimeter cup through the metal bar.
What is heat or energy?
The specific heat capacities of 3 substances are given below. Each one has a mass of 20g. For the following questions use the equation for heat:
Q=mcDeltaT
Ice = 0.5 cal/goC
Copper = .09 cal/goC
Wood = .45 cal/goC
If 1450 calories of heat were applied to each substance, this one would change temperature most and by this much.
What is copper by 805.6oC?
A graduated cylinder is filled with 15 mL of water. When a metal bar with a mass of 78 g is placed in the graduated cylinder the water level rises to 28 mL. This is the density of the metal bar.
What is 6 g/cm3?
Label the following statements on the graph below:
1. The H2O gas is being cooled in a beaker by ice on the outside of the beaker.
2. Most of the H2O gas has condensed.
3. About half of the H2O has turned to ice.
4. The H2O is at about 30oC
What is
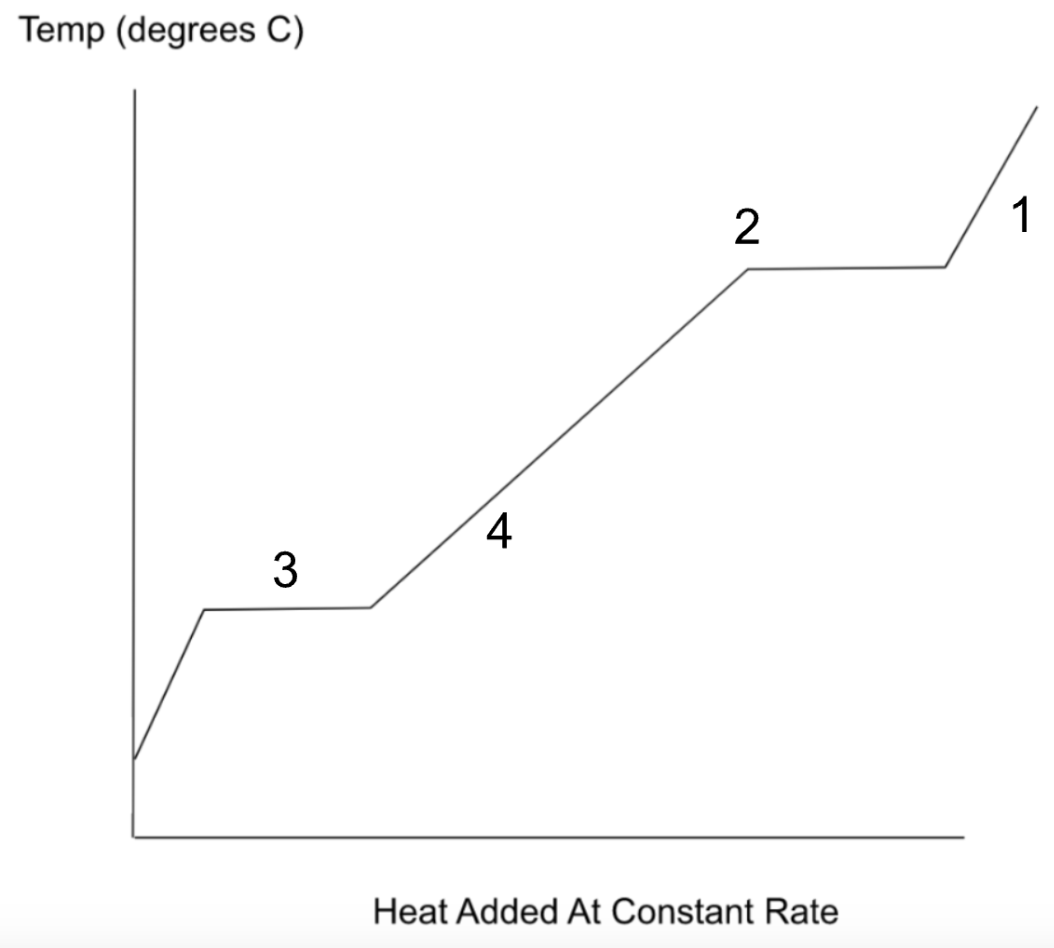
What is the Celsius scale, and what is water's density = 1 g/mL, and what is water's specific heat = 1 cal/goC?