What is the proper name for the following groups on the periodic table:
a) Group 1
b) Group 2
c) Groups 3 - 12
d) Group 17
e) Group 18
a) Alkali Metals
b) Alkaline Earth Metals
c) Transition Metals
d) Halogens
e) Noble Gases
Who discovered the Periodic Table?
Dmitri Mendeleev
Manganese (II) sulfate is a common fertilizer used in agriculture. What is the chemical formula for manganese (II) sulfate?
MnSO₄
What type of intermolecular forces is observed in the following substance:
CBr4
London Dispersion Forces
The Mentos and Coke experiment is this type of change for this reason
The mentos is a rough candy and provides a nucleation point of the carbonation already dissolved in the Coke. This makes the mentos and Coke experiment a physical change.
Why must equations be balanced?
So they obey the law of conservation of matter
Who developed each of the following models and what is the limits and merits for the planetary model?
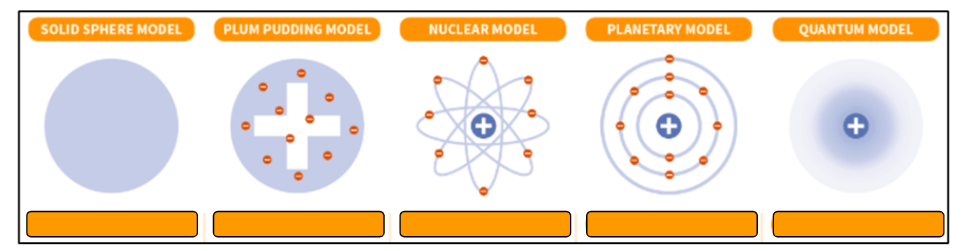
Solid Sphere - Dalton/Democrateous
Plum Pudding Model - J. J. Thompson
Nuclear Model - Rutherford
Planetary Model - Bohr
Quantum Model - Schrodinger/Heisenburg
The Bohr model finally shows us that electrons exist in orbitals (merit) but their placement isn't 100% correct (limit)
Which element has the largest atomic radius?
a. lithium (Li)
b.potassium (K)
c. beryllium (Be)
d. carbon (C)
b.potassium (K)
Describe how metals bond
The delocalized electrons and the metal cation are electrostatically attracted to one another. This holds the atoms in a regular structure through metallic bonding.
Mercury is toxin to humans and animals and, the more that is ingested, the more likely it is to cause harm to the individual. Describe the properties of mercury
Chemical, Reactive, Extensive
Why do we use baking soda instead of starch to make cakes fluffy?
Starch does not react with water or acid to make bubbles. Bubbles are what make cakes fluffy. Baking, soda, however, will make bubbles
For the following chemical reactions, balance it and determine the reaction type.
___ Fe + ___ O2 → ____ Fe2O3
4 Fe + 3 O2 → 2 Fe2O3
Synthesis
What is the electron configuration (using noble gas notation) of the oxygen ion?
[He]2s22p6
What category of elements has the property of being malleable and ductile?
Metals
Students in class argue about whether salt (NaCl) or water (H2O) has stronger bonding. Who does and why?
Salt never melts and water has a lower boiling point, so salt has stronger bonding.
Under what conditions will an object float on water? Be specific.
The object must be less dense (less than 1.0 g/mL) than water
One pollutant produced in automobile engines is nitrogen dioxide (NO2). It changes to form nitrogen oxide (NO) and oxygen gas when exposed to sunlight. Of what reaction type is this an example?
This is a decomposition reaction
For the following chemical reaction, predict the products, balance it and determine the reaction type.
____ AgNO3 + ____ Fe2(SO3)3 →
6 AgNO3 + 1 Fe2(SO3)3 → 3 Ag2SO3 + 2 Fe(NO3)3
Double Displacement
Double Replacement
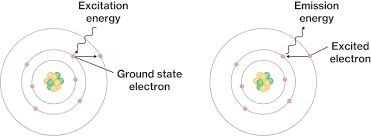
This is why copper appears blue when burned and a diagram that depicts this phenomenon
Because as electrons fall back from an excited state to ground state, they emit light.
Why does electronegativity increase going across a period but not down a group?
As you move across a period, the elements are closer to having a full outer shell and the electrostatic attraction between the valence electrons and the nucleus is stronger, making it easier for them to pull in more electrons. However, as you go down a group, the elements are increasing in size and the valence electrons are further away from the nucleus, making it more difficult to pull in new electrons.
Rust forms when iron is left out in the presence of oxygen. The chemical formula for rust is Fe2O3. What is its chemical name?
Iron (III) oxide
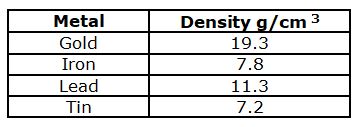
A sample of an unknown substance has a mass of 28.8 grams and a volume of 4 cm3. Based on it’s density, it could be which of the following substances?
Tin
Model 1 CO2(l) → CO2(g)
Model 2 CO2 (g) → C (s) + O2(g)
Model 1 represents this kind of change
Physical Change
(going from a liquid to a gas is a phase change, and thus a physical change)
Predict the products and balance the following chemical equation:
___C3H8 + ___ O2 →
C3H8 + 5 O2 → 3CO2 + 4H2O
The average atomic mass for element X and identity is

28.08 amu, Silicon
How is the trend in ionization energy related to the trend in atomic radii? What impact does it have on ionization energy?
Ionization energy increases as you move closer to the noble gasses because you get closer to having a full octet of electrons. This also reduces the atomic radius because the electrostatic attraction between the nucleus and the valence electrons is greater, drawing the valence electrons closer to the nucleus, making the atom appear smaller.
A student places two substances into a Bunsen burner. Substance A melts and then ignites. Substance B does not change. Which substance is covalently bonded and why?
Substance A because it has a relatively low melting point.
Many covalent substances are volatile liquids or gases at room temperature because
the intermolecular forces of attraction holding the molecules together are weak.
You are planning an investigation of the physical and chemical properties of sodium (Na) and water. You do a little research and find that a reaction occurs between the two in the reaction seen below.
Na (s) + H2O (l) → NaOH (aq) + H2 (g) + heat
At the end of the experiment, you notice that the total mass after the reaction is complete is less than the initial mass. This observation can be explained by
The loss of H2 gas
Predict the products and balance the following reaction:
___ Mg + ____ NaI →
No Reaction
Fluorine’s electron configuration is 1s22s22p5 and it is a very reactive nonmetal. Which of the following elements do you think will exhibit properties similar to fluorine?
a) 1s22s22p4
b) 1s22s22p6
c) 1s22s22p63s2
d) 1s22s22p63s23p5
d) 1s22s22p63s23p5
1s22s22p5 is Fluorine, which is in group 17
1s22s22p63s23p5 is chlorine, which is also in group 17, meaning they have similar properties
How do you know that potassium, an alkali metal, is highly reactive?
It has one valence electron.
Hydrogen thiocyanate is a very corrosive acid. What is the chemical formula for hydrogen thiocyanate?
HSCN
Which of the following will have the highest boiling point: HF, HBr, HI, HCl
HF has hydrogen bonding
All of the others are dipole-dipole interactions
Which means HF will have the highest BP because it has the strongest IMFs
A thin strip of iron with a mass of 15.5g is placed into a solution containing 21.0g of copper (II) sulfate and copper begins to form. After some times, the reactions stops because all the copper (II) sulfate has reacted. The iron strip is found to have a mass of 8.5g. The mass of copper formed is found to be 8.60g. What mass of iron (II) sulfate has been formed in the reaction?
Mass of iron = Initial mass – Final mass
= 15.5 – 8.5 = 7.0g
Mass of copper (II) sulfate = 21.0g
Mass of copper = 8.60g
According to law of conservation of mass,
Mass of iron + Mass of copper (II) sulfate = Mass of copper + Mass of iron (II) sulfate
7.0g + 21.0g = 8.60g + Mass of iron (II) sulfate
So, Mass of iron (II) sulfate = 19.40g
Predict the products and balance the following chemical reaction:
____ AlN + ____ Ca(OH)2 →
2AlN + 3 Ca(OH)2 → 2 Al(OH)3 + Ca3N2