This subatomic particle has a positive charge.
Proton
Indivisible atoms was an essential part of whose atomic theory?
Dalton
Avogadro’s number (6.022x1023 mol-1) tells us
The number of items in a mole
How do we know if a formula is an empirical formula or not?
The empirical formula will always have the subscripts divided by the greatest common factor (it is no longer able to be simplified)
Bromine has just two major isotopes (Br-81 and Br-79) giving it an atomic mass of 79.904 amu. Based on this information, which of the following statements can explain the atomic mass value?
Since 80 is the average of 81 & 79 they are in equal proportions
SO....Br-79 and Br-81 exist in about equal proportions.
The smallest particle that retains an element’s identity.
atom
Rutherford’s gold foil experiment led to the discovery of what?
nucleus
How many atoms of lead are present in 2.46 mol of lead?

What is the mass, in grams, of 1.24 mol of oxygen atoms?
1.24 mol of O x 16 g of Oxygen= 19.8g O
1 mol O
An isotope of an element has a different mass because it has a different number of…
neutrons
Bottom number of the isotopes numbers (the atomic number) is the number of what two things?
protons or electrons
Which two subatomic particles contribute to an atom’s charge?
electrons and protons
The molar mass of oxygen atoms.
16g/mol
What % of water is made up of O?
find the atomic mass of the molecule by multiplying its mass by the subscript for each element and then adding the masses together
Take the mass found for the element you are looking at and divide it by the atomic mass of the compound and multiply by 100
O: 16
H: 1*2 = 2
water = 18g/mol
16/18 * 100 = 89%
If you are given all the known isotopes' abundance and atomic mass, how do you calculate the average atomic mass?
Divide the abundance percent by 100%
multiply the abundance by the atomic mass for each isotope
Add the answers together to get the average atomic mass
How do you determine the number of neutrons?
Top number of the two numbers of an element is the mass number. Number of neutrons = mass number – atomic number
Describe Rutherford's gold foil experiment and what he observed.
Ernest Rutherford shot α particles at a thin sheet of gold foil and observed the pattern of scatter of the particles.
Rutherford observed that most of the particles passed through the foil with no deflection, and a small fraction were deflected at large angles or reflected directly back.
The number of moles in 22 g of CO2.
22g CO2 x 1 mol CO2 = 0.5mol CO2
44g CO2
How to determine the empirical formula:
Change the % to grams for each element by assuming __________of the compound
Convert the grams to moles using the _________ _________ for each element
Determine the _____________ ____________ (the reactant that will be used up first)
Divide each molar quantity found by the _________________ _______________'s _____________.
Multiply each mole by a lowest common denominator to make the mole a ____________ number
These moles are the new _________________.
How to determine the empirical formula:
Change the % to grams for each element by assuming 100g total of the compound
Convert the grams to moles using the molar mass for each element
Determine the limiting reactant (the reactant that will be used up first)
Divide each molar quantity found by the limiting reactant’s moles
Multiply each mole by a lowest common denominator to make the mole a whole number
These moles are the new subscripts.
How many isotopes are shown in this graph?
What are the % abundance and mass of each isotope?
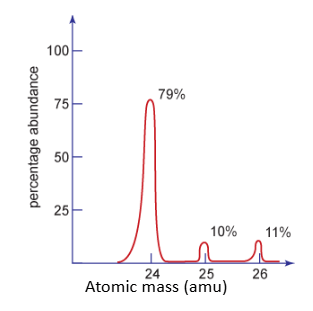
3
79% -24
10% - 25
11% - 26
Which of two subatomic particles contribute to an atom’s mass?
protons and neutrons
What was Rutherford able to conclude about the nucleus from his gold foil experiment?
He concluded that the nucleus is tiny and densely packed compared with the atom as a whole.
He proposed that the atom is mostly empty space. This allowed him to determine a model for the atom which is now known as the nuclear atom.
How many atoms are in 3.4 mol of Na?
3.4 mol x 6.022 x 1023 Na atoms
1 mol Na
= 20.4748 x 1023 atoms of Na
=2.0 x 1024 atoms Na
If you are given the EFM and the molar mass how can you determine the molecular formula?
Divide the molar mass by the efm:
Molar mass
Efm
Multiply formula subscripts by this number
Using the periodic table, find the following quantities for fluorine (F):
Atomic number:
Atomic weight:
Number of protons:
Number of electrons:
Number of neutrons:
Atomic number: 9
Atomic weight: 18.998
Number of protons: 9
Number of electrons: 9
Number of neutrons: 18.998-9 = 10